Rostral ventromedial medulla neurons that project to the spinal cord express multiple opioid receptor phenotypes
- PMID: 12486178
- PMCID: PMC6758433
- DOI: 10.1523/JNEUROSCI.22-24-10847.2002
Rostral ventromedial medulla neurons that project to the spinal cord express multiple opioid receptor phenotypes
Abstract
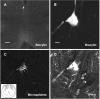
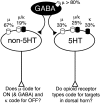
The rostral ventromedial medulla (RVM) forms part of a descending pathway that modulates nociceptive neurotransmission at the level of the spinal cord dorsal horn. However, the involvement of descending RVM systems in opioid analgesia are a matter of some debate. In the present study, patch-clamp recordings of RVM neurons were made from rats that had received retrograde tracer injections into the spinal cord. More than 90% of identified spinally projecting RVM neurons responded to opioid agonists. Of these neurons, 53% responded only to the mu-opioid agonist D-Ala2, N-Me-Phe4, Gly-ol5 enkephalin, 14% responded only to the kappa-opioid agonist U-69593, and another group responded to both mu and kappa opioids (23%). In unidentified RVM neurons, a larger proportion of neurons responded only to mu opioids (75%), with smaller proportions of kappa- (4%) and mu/kappa-opioid (13%) responders. These RVM slices were then immunostained for tryptophan hydroxylase (TPH), a marker of serotonergic neurons. Forty-percent of spinally projecting neurons and 11% of unidentified neurons were TPH positive. Of the TPH-positive spinally projecting neurons, there were similar proportions of mu- (33%), kappa- (25%), and mu/kappa-opioid (33%) responders. Most of the TPH-negative spinally projecting neurons were mu-opioid responders (67%). These findings indicate that functional opioid receptor subtypes exist on spinally projecting serotonergic and nonserotonergic RVM neurons. The proportions of mu- and kappa-opioid receptors expressed differ between serotonergic and nonserotonergic neurons and between retrogradely labeled and unlabeled RVM neurons. We conclude that important roles exist for both serotonergic and nonserotonergic RVM neurons in the mediation of opioid effects.
Figures






References
-
- Ackley MA, Hurley RW, Virnich DE, Hammond DL. A cellular mechanism for the antinociceptive effect of a kappa opioid receptor agonist. Pain. 2001;91:377–388. - PubMed
-
- Azami J, Llewelyn MB, Roberts MH. The contribution of nucleus reticularis paragigantocellularis and nucleus raphe magnus to the analgesia produced by systemically administered morphine, investigated with the microinjection technique. Pain. 1982;12:229–246. - PubMed
-
- Bederson JB, Fields HL, Barbaro NM. Hyperalgesia during naloxone-precipitated withdrawal from morphine is associated with increased on-cell activity in the rostral ventromedial medulla. Somatosens Mot Res. 1990;7:185–203. - PubMed
-
- Christie MJ. Do medullary serotonergic neurons tonically modulate nociceptive transmission. Pain Forum. 1998;7:155–158.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
