Induction of hippocampal long-term potentiation during waking leads to increased extrahippocampal zif-268 expression during ensuing rapid-eye-movement sleep
- PMID: 12486186
- PMCID: PMC6758456
- DOI: 10.1523/JNEUROSCI.22-24-10914.2002
Induction of hippocampal long-term potentiation during waking leads to increased extrahippocampal zif-268 expression during ensuing rapid-eye-movement sleep
Abstract
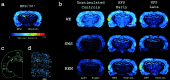
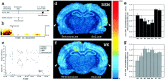
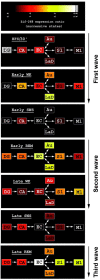
Rapid-eye-movement (REM) sleep plays a key role in the consolidation of memories acquired during waking (WK). The search for mechanisms underlying that role has revealed significant correlations in the patterns of neuronal firing, regional blood flow, and expression of the activity-dependent gene zif-268 between WK and subsequent REM sleep. Zif-268 integrates a major calcium signal transduction pathway and is implicated by several lines of evidence in activity-dependent synaptic plasticity. Here we report that the induction of hippocampal long-term potentiation (LTP) during WK in rats leads to an upregulation of zif-268 gene expression in extrahippocampal regions during subsequent REM sleep episodes. This upregulation occurs predominantly in the amygdala, entorhinal, and auditory cerebral cortices during the first REM sleep episodes after LTP induction and reaches somatosensory and motor cerebral cortices as REM sleep recurs. We also show that hippocampal inactivation during REM sleep blocks extrahippocampal zif-268 upregulation, indicating that cortical and amygdalar zif-268 expression during REM sleep is under hippocampal control. Thus, expression of an activity-dependent gene involved in synaptic plasticity propagates gradually from the hippocampus to extrahippocampal regions as REM sleep recurs. These findings suggest that a progressive disengagement of the hippocampus and engagement of the cerebral cortex and amygdala occurs during REM sleep. They are also consistent with the view that REM sleep constitutes a privileged window for hippocampus-driven cortical activation, which may play an instructive role in the communication of memory traces from the hippocampus to the cerebral cortex.
Figures



References
-
- Ben-Ari Y, Le Gal la Salle G. Plasticity at unitary level. II. Modifications during sensory-sensory association procedures. Electroencephalogr Clin Neurophysiol. 1972;32:667–679. - PubMed
-
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
