Attenuation of nicotine-induced antinociception, rewarding effects, and dependence in mu-opioid receptor knock-out mice
- PMID: 12486188
- PMCID: PMC6758457
- DOI: 10.1523/JNEUROSCI.22-24-10935.2002
Attenuation of nicotine-induced antinociception, rewarding effects, and dependence in mu-opioid receptor knock-out mice
Abstract
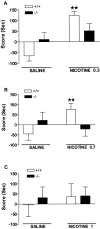
The involvement of mu-opioid receptors in different behavioral responses elicited by nicotine was explored by using mu-opioid receptor knock-out mice. The acute antinociceptive responses induced by nicotine in the tail-immersion and hot-plate tests were reduced in the mutant mice, whereas no difference between genotypes was observed in the locomotor responses. The rewarding effects induced by nicotine were then investigated using the conditioning place-preference paradigm. Nicotine produced rewarding responses in wild-type mice but failed to produce place preference in knock-out mice, indicating the inability of this drug to induce rewarding effects in the absence of mu-opioid receptors. Finally, the somatic expression of the nicotine withdrawal syndrome, precipitated in dependent mice by the injection of mecamylamine, was evaluated. Nicotine withdrawal was significantly attenuated in knock-out mutants when compared with wild-type mice. In summary, the present results show that mu-opioid receptors are involved in the rewarding responses induced by nicotine and participate in its antinociceptive responses and the expression of nicotine physical dependence.
Figures



References
-
- Almeida LE, Pereira EF, Alkondon M, Fawcett WP, Randall WR, Albuquerque EX. The opioid antagonist naltrexone inhibits activity and alters expression of α7 and α4β2 nicotinic receptors in hippocampal neurons: implications for smoking cessation programs. Neuropharmacology. 2000;39:2740–2755. - PubMed
-
- Balfour DJ. The effects of nicotine on brain neurotransmitter systems. Pharmacol Ther. 1982;16:269–282. - PubMed
-
- Castañé A, Valjent E, Ledent C, Parmentier M, Maldonado R, Valverde O. Lack of CB1 cannabinoid receptors modifies nicotine behavioural responses, but not nicotine abstinence. Neuropharmacology. 2002;43:857–867. - PubMed
-
- Corrigall WA, Coen KM. Opiate antagonists reduce cocaine but not nicotine self-administration. Psychopharmacology. 1991;104:167–170. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
