Feedforward mechanisms of excitatory and inhibitory cortical receptive fields
- PMID: 12486192
- PMCID: PMC6758449
- DOI: 10.1523/JNEUROSCI.22-24-10966.2002
Feedforward mechanisms of excitatory and inhibitory cortical receptive fields
Abstract
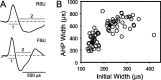
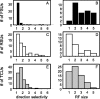
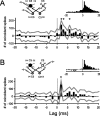
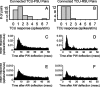
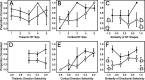
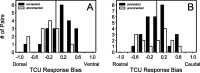
Excitatory and inhibitory cortical layer IV neurons have distinctive response properties. Thalamocortical connectivity that may underlie differences was examined using cross-correlation analyses of pairs of thalamic and cortical neurons in the rat whisker/barrel system. Cortical layer IV cells discharging fast spikes, presumed inhibitory neurons, were distinguished from regular-spike units, presumed excitatory neurons, by the extracellular waveform shape. Regular-spike neurons fired less robustly and had smaller receptive fields (RFs) and greater directional tuning than fast-spike cells. Presumed excitatory neurons were less likely to receive thalamocortical connections, and their connections were, on average, weaker. RF properties of thalamic inputs to both cell types were equivalent, except that the most highly responsive thalamic cells contacted only fast-spike neurons. In contrast, the size and directional tuning of cortical RFs were related to the number of detectable thalamocortical inputs. Connected thalamocortical pairs were likely to have matching RF characteristics. The smaller, more directionally selective RFs of excitatory neurons may be a consequence of their weaker net thalamic drive, their more nonlinear firing characteristics and pervasive feedforward inhibition provided by strongly driven, broadly tuned inhibitory neurons.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
