Interaction of excitation and inhibition in anteroventral cochlear nucleus neurons that receive large endbulb synaptic endings
- PMID: 12486196
- PMCID: PMC6758445
- DOI: 10.1523/JNEUROSCI.22-24-11004.2002
Interaction of excitation and inhibition in anteroventral cochlear nucleus neurons that receive large endbulb synaptic endings
Abstract
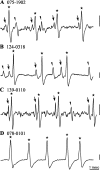
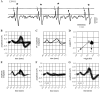
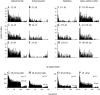
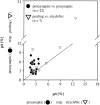
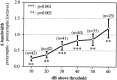
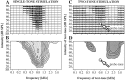
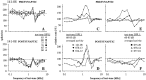
Spherical bushy cells (SBCs) of the anteroventral cochlear nucleus (AVCN) receive their main excitatory input from auditory nerve fibers (ANFs) through large synapses, endbulbs of Held. These cells are also the target of inhibitory inputs whose function is not well understood. The present study examines the role of inhibition in the encoding of low-frequency sounds in the gerbil's AVCN. The presynaptic action potentials of endbulb terminals and postsynaptic action potentials of SBCs were monitored simultaneously in extracellular single-unit recordings in vivo. An input-output analysis of presynaptic and postsynaptic activity was performed for both spontaneous and acoustically driven activity. Two-tone stimulation and neuropharmacological experiments allowed the effects of neuronal inhibition and cochlear suppression on SBC activity to be distinguished. Ninety-one percent of SBCs showed significant neuronal inhibition. Inhibitory sidebands enclosed the high- or low-frequency, or both, sides of the excitatory areas of these units; this was reflected as a presynaptic to postsynaptic increase in frequency selectivity of up to one octave. Inhibition also affected the level-dependent responses at the characteristic frequency. Although in all units the presynaptic recordings showed monotonic rate-level functions, this was the case in only half of the postsynaptic recordings. In the other half of SBCs, postsynaptic inhibitory areas overlapped the excitatory areas, resulting in nonmonotonic rate-level functions. The results demonstrate that the sound-evoked spike activity of SBCs reflects the integration of acoustically driven excitatory and inhibitory input. The inhibition specifically affects the processing of the spectral, temporal, and intensity cues of acoustic signals.
Figures





References
-
- Abeles M, Goldstein MH. Multispike train analysis. Proc IEEE. 1977;65:762–773.
-
- Adams JC. Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem. 1981;29:775. - PubMed
-
- Bazwinsky I, Härtig W, Rübsamen R. Distribution of different calcium binding proteins in the cochlear nucleus of gerbil and opossum (Abstract 260). Presented at Midwinter Meeting of the Association for Research in Otolaryngology. St. Petersburg Beach, FL, February; 1999.
-
- Blackburn CC, Sachs MB. Classification of unit types in the anteroventral cochlear nucleus: PST histograms and regularity analysis. J Neurophysiol. 1989;62:1303–1329. - PubMed
-
- Brawer JR, Morest DK. Relations between auditory nerve endings and cell types in the cat's anteroventral cochlear nucleus seen with the Golgi method and Nomarski optics. J Comp Neurol. 1975;160:491–506. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
