High-resolution analysis of ethanol-induced locomotor stimulation in Drosophila
- PMID: 12486199
- PMCID: PMC6758417
- DOI: 10.1523/JNEUROSCI.22-24-11035.2002
High-resolution analysis of ethanol-induced locomotor stimulation in Drosophila
Abstract
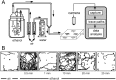
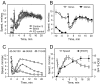
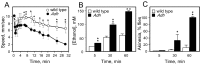
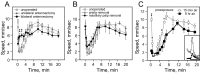
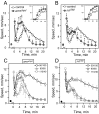
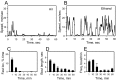
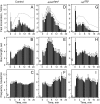
Understanding how ethanol influences behavior is key to deciphering the mechanisms of ethanol action and alcoholism. In mammals, low doses of ethanol stimulate locomotion, whereas high doses depress it. The acute stimulant effect of ethanol has been proposed to be a manifestation of its rewarding effects. In Drosophila, ethanol exposure transiently potentiates locomotor activity in a biphasic dose- and time-dependent manner. An initial short-lived peak of activity corresponds to an olfactory response to ethanol. A second, longer-lasting period of increased activity coincides with rising internal ethanol concentrations; these closely parallel concentrations that stimulate locomotion in mammals. High-resolution analysis of the walking pattern of individual flies revealed that locomotion consists of bouts of activity; bout structure can be quantified by bout frequency, bout length, and the time spent walking at high speeds. Ethanol exposure induces both dramatic and dynamic changes in bout structure. Mutants with increased ethanol sensitivity show distinct changes in ethanol-induced locomotor behavior, as well as genotype-specific changes in activity bout structure. Thus, the overall effect of ethanol on locomotor behavior in Drosophila is caused by changes in discrete quantifiable parameters of walking pattern. The effects of ethanol on locomotion are comparable in flies and mammals, suggesting that Drosophila is a suitable model system to study the underlying mechanisms.
Figures
References
-
- Aceves PE, Booker R, Duerr JS, Livingstone MS, Quinn WG, Smith RF, Sziber PP, Tempel BL, Tully TP. Learning and memory in Drosophila, studied with mutants. Cold Spring Harb Symp Quant Biol. 1983;2:831–840. - PubMed
-
- Bainton RJ, Tsai LT-Y, Singh CM, Moore MS, Neckameyer WS, Heberlein U. Dopamine modulates acute responses to cocaine, nicotine, and ethanol in Drosophila. Curr Biol. 2000;10:187–194. - PubMed
-
- Cunningham CL, Howard MA, Gill SJ, Rubinstein M, Low MJ, Grandy DK. Ethanol-conditioned place preference is reduced in dopamine D2 receptor-deficient mice. Pharmacol Biochem Behav. 2000;67:693–699. - PubMed
-
- de Bruyne M, Foster K, Carlson JR. Odor coding in the Drosophila antenna. Neuron. 2001;30:537–552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
