Electroconvulsive seizure behavior in Drosophila: analysis of the physiological repertoire underlying a stereotyped action pattern in bang-sensitive mutants
- PMID: 12486202
- PMCID: PMC6758420
- DOI: 10.1523/JNEUROSCI.22-24-11065.2002
Electroconvulsive seizure behavior in Drosophila: analysis of the physiological repertoire underlying a stereotyped action pattern in bang-sensitive mutants
Abstract
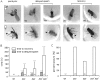
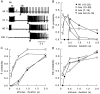
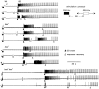
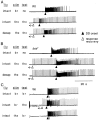
Drosophila bang-sensitive mutants display a remarkable stereotyped behavioral sequence during mechanical disturbances. This seizure repertoire consists of initial and delayed bouts of spasm interposed with paralysis and followed by recovery of activity and a period of refractoriness to further stimulation. Electroconvulsive stimuli across the brain induced a similar seizure behavior in tethered flies, in which corresponding electrophysiological events could be readily recorded in indirect flight muscles [dorsal longitudinal muscles (DLMs)] of the giant fiber (GF) pathway. The DLM physiological repertoire consisted of initial and delayed discharges (IDs and DDs), a response failure and recovery, followed by a refractory period. Interestingly, wild-type flies also displayed the same electroconvulsive repertoire, albeit inducible only at higher stimulus intensities and with briefer expression. The DLM repertoire presumably originated from activities of distinct neural circuits subserving normal function and reflected the general sequence of excitation and depression of the nervous system as a whole, as shown by simultaneous recordings along the different body axes. The well characterized GF pathway facilitated localization of circuits responsible for response failure and ID and DD motor patterns by surgical manipulations, recording-stimulating site analysis, and genetic mosaic studies. A flight pattern generator is most likely the major contributor to shaping the DD pattern, with modifications by active integration of individual motor neurons and associated interneurons. The robust electroconvulsive repertoire of DLMs provides a convenient window for further genetic analysis of the interacting neural mechanisms underlying a stereotyped action pattern in Drosophila, which shows striking parallels with aspects of seizure in mammalian species.
Figures








References
-
- Baird DH, Koto M, Wyman RJ. Dendritic reduction in Passover, a Drosophila mutant with a defective giant fiber neuronal pathway. J Neurobiol. 1993;24:971–984. - PubMed
-
- Benzer S. From the gene to behavior. JAMA. 1971;218:1015–1022. - PubMed
-
- Blagburn JM, Alexopoulos H, Davies JA, Bacon JP. Null mutation in shaking-B eliminates electrical, but not chemical, synapses in the Drosophila giant fiber system: a structural study. J Comp Neurol. 1999;404:449–458. - PubMed
-
- Burg MG. PhD thesis. University of Iowa; 1987. Genetic and mosaic analysis of mutations which alter nerve and muscle excitability in Drosophila melanogaster: effects on development and behavior.
-
- Burg MG, Wu C-F. A class of Drosophila behavioral mutants that are sensitive to both mechanical vibrations and temperature conditions. Soc Neurosci Abstr. 1987;13:619.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
