Engineered calmodulins reveal the unexpected eminence of Ca2+ channel inactivation in controlling heart excitation
- PMID: 12486220
- PMCID: PMC139290
- DOI: 10.1073/pnas.262372999
Engineered calmodulins reveal the unexpected eminence of Ca2+ channel inactivation in controlling heart excitation
Abstract
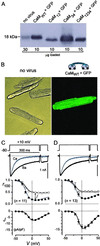
Engineered calmodulins (CaMs), rendered Ca2+-insensitive by mutations, function as dominant negatives in heterologous systems, and have revealed mechanisms of ion channel modulation by Ca2+/CaM. The use of these CaMs in native mammalian cells now emerges as a strategy to unmask the biology of such Ca2+ feedback. Here, we developed recombinant adenoviruses bearing engineered CaMs to facilitate their expression in adult heart cells, where Ca2+ regulation may be essential for moment-to-moment control of the heartbeat. Engineered CaMs not only eliminated the Ca2+-dependent inactivation of native calcium channels, but exposed an unexpectedly large impact of removing such feedback: the unprecedented (4- to 5-fold) prolongation of action potentials. This striking result recasts the basic paradigm for action-potential control and illustrates the promise of virally delivered engineered CaM to investigate the biology of numerous other CaM-signaling pathways.
Figures



References
-
- Jurado L. A., Chockalingam, P. S. & Jarrett, H. W. (1999) Physiol. Rev. 79, 661-682. - PubMed
-
- DeMaria C. D., Soong, T. W., Alseikhan, B. A., Alvania, R. S. & Yue, D. T. (2001) Nature 411, 484-489. - PubMed
-
- Hoeflich K. P. & Ikura, M. (2002) Cell 108, 739-742. - PubMed
-
- Dasgupta M., Honeycutt, T. & Blumenthal, D. K. (1989) J. Biol. Chem. 264, 17156-17163. - PubMed
-
- Imredy J. P. & Yue, D. T. (1994) Neuron 12, 1301-1318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

