Photoresponsive polymer-enzyme switches
- PMID: 12486222
- PMCID: PMC139188
- DOI: 10.1073/pnas.262427799
Photoresponsive polymer-enzyme switches
Abstract
The ability to photoregulate enzyme activities could provide important new opportunities for development of diagnostic assays, sequential bioprocessing, and lab assays in both traditional and microfluidic formats. We show here that the photoinduced changes in the size and hydration of a "smart" polymer chain coil can be used to regulate substrate access and enzyme activity when conjugated to the enzyme at a specific point just outside the active site. The photoresponsive polymers thus serve jointly as antennae and actuators that reversibly respond to distinct optical signals to switch the polymer-enzyme conjugates on and off, and work when the conjugate is free in solution or when immobilized on magnetic beads.
Figures
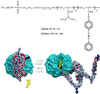
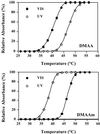
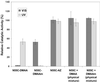


References
-
- Haupt W. (1983) Philos. Trans. R. Soc. London B 303, 467-478.
-
- Smith H., (1975) Photochrome and Photomorphogenesis (McGraw-Hill, London), pp. 22.
-
- Feher G., Allen, J. P., Okamura, M. Y. & Rees, D. C. (1989) Nature 339, 111-116.
-
- Stryer L. (1986) Am. Rev. Neurosci. 9, 87-119. - PubMed
-
- Willner I. & Rubin, S. (1996) Angew. Chem. Int. Ed. Engl. 35, 367-385.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

