Z-DNA-binding proteins can act as potent effectors of gene expression in vivo
- PMID: 12486233
- PMCID: PMC139201
- DOI: 10.1073/pnas.262672699
Z-DNA-binding proteins can act as potent effectors of gene expression in vivo
Abstract
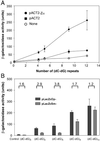
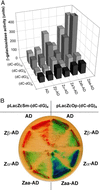
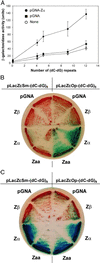
The role of Z-DNA-binding proteins in vivo is explored in yeast. A conformation-specific yeast one-hybrid system is made in which formation of Z-DNA is studied near a minimal promoter site where it can be stabilized by negative supercoiling in addition to protein binding. Experiments were carried out with a Z-DNA-binding protein domain from the editing enzyme, double-stranded RNA adenosine deaminase 1. In the one-hybrid system, the reporter gene is activated when a Z-DNA-specific binding domain is fused with an activation domain and expressed in vivo. Significantly, it was found that even in the absence of the activation domain there is substantial transcription of the reporter gene if the Z-DNA-binding protein is expressed in the cell. This result suggests that Z-DNA formation in the promoter region induced or stabilized by a Z-DNA-binding protein can act as a cis-element in gene regulation. Related results have been found recently when the human chromatin-remodeling system converts a segment of DNA in the promoter region of the human colony-stimulating factor 1 gene into the left-handed Z-conformation.
Figures

References
-
- Liu J., Wilson, T. E., Milbrandt, J. & Johnston, M. (1993) Methods 5, 125-137.
-
- Allen J. B., Walberg, M. W., Edwards, M. C. & Elledge, S. J. (1995) Trends Biochem. Sci. 20, 511-516. - PubMed
-
- Wang A. H., Quigley, G. J., Kolpak, F. J., Crawford, J. L., van Boom, J. H., van der Marel, G. & Rich, A. (1979) Nature 282, 680-686. - PubMed
-
- Rich A., Nordheim, A. & Wang, A. H. (1984) Annu. Rev. Biochem. 53, 791-846. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

