Hyperinsulinism induced by targeted suppression of beta cell KATP channels
- PMID: 12486236
- PMCID: PMC139257
- DOI: 10.1073/pnas.012479199
Hyperinsulinism induced by targeted suppression of beta cell KATP channels
Abstract
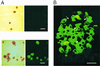
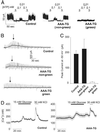
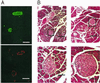
ATP-sensitive K+ (K(ATP)) channels couple cell metabolism to electrical activity. To probe the role of K(ATP) in glucose-induced insulin secretion, we have generated transgenic mice expressing a dominant-negative, GFP-tagged K(ATP) channel subunit in which residues 132-134 (Gly-Tyr-Gly) in the selectivity filter were replaced by Ala-Ala-Ala, under control of the insulin promoter. Transgene expression was confirmed by both beta cell-specific green fluorescence and complete suppression of channel activity in those cells ( approximately 70%) that did fluoresce. Transgenic mice developed normally with no increased mortality and displayed normal body weight, blood glucose levels, and islet architecture. However, hyperinsulinism was evident in adult mice as (i) a disproportionately high level of circulating serum insulin for a given glucose concentration ( approximately 2-fold increase in blood insulin), (ii) enhanced glucose-induced insulin release from isolated islets, and (iii) mild yet significant enhancement in glucose tolerance. Enhanced glucose-induced insulin secretion results from both increased glucose sensitivity and increased release at saturating glucose concentration. The results suggest that incomplete suppression of K(ATP) channel activity can give rise to a maintained hyperinsulinism.
Figures


References
-
- Cook D. L., Satin, L. S., Ashford, M. L. & Hales, C. N. (1988) Diabetes 37, 495-498. - PubMed
-
- Misler S. & Giebisch, G. (1992) Curr. Opin. Nephrol. Hypertens. 1, 21-33. - PubMed
-
- Huopio H., Shyng, S. L., Otonkoski, T. & Nichols, C. G. (2002) Am. J. Physiol. 283, E207-E216. - PubMed
-
- Gerich J. E. (1985) Mayo Clin. Proc. 60, 439-443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

