Genes for the cytoskeletal protein tubulin in the bacterial genus Prosthecobacter
- PMID: 12486237
- PMCID: PMC139267
- DOI: 10.1073/pnas.012516899
Genes for the cytoskeletal protein tubulin in the bacterial genus Prosthecobacter
Abstract
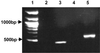
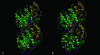
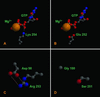
Tubulins, the protein constituents of the microtubule cytoskeleton, are present in all known eukaryotes but have never been found in the Bacteria or Archaea. Here we report the presence of two tubulin-like genes [bacterial tubulin a (btuba) and bacterial tubulin b (btubb)] in bacteria of the genus Prosthecobacter (Division Verrucomicrobia). In this study, we investigated the organization and expression of these genes and conducted a comparative analysis of the bacterial and eukaryotic protein sequences, focusing on their phylogeny and 3D structures. The btuba and btubb genes are arranged as adjacent loci within the genome along with a kinesin light chain gene homolog. RT-PCR experiments indicate that these three genes are cotranscribed, and a probable promoter was identified upstream of btuba. On the basis of comparative modeling data, we predict that the Prosthecobacter tubulins are monomeric, unlike eukaryotic alpha and beta tubulins, which form dimers and are therefore unlikely to form microtubule-like structures. Phylogenetic analyses indicate that the Prosthecobacter tubulins are quite divergent and do not support recent horizontal transfer of the genes from a eukaryote. The discovery of genes for tubulin in a bacterial genus may offer new insights into the evolution of the cytoskeleton.
Figures
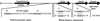

References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

