Effects of strand and directional asymmetry on base-base coupling and charge transfer in double-helical DNA
- PMID: 12486238
- PMCID: PMC139180
- DOI: 10.1073/pnas.012669599
Effects of strand and directional asymmetry on base-base coupling and charge transfer in double-helical DNA
Abstract
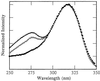
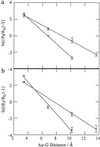
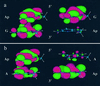
Mechanistic models of charge transfer (CT) in macromolecules often focus on CT energetics and distance as the chief parameters governing CT rates and efficiencies. However, in DNA, features unique to the DNA molecule, in particular, the structure and dynamics of the DNA base stack, also have a dramatic impact on CT. Here we probe the influence of subtle structural variations on base-base CT within a DNA duplex by examining photoinduced quenching of 2-aminopurine (Ap) as a result of hole transfer (HT) to guanine (G). Photoexcited Ap is used as a dual reporter of variations in base stacking and CT efficiency. Significantly, the unique features of DNA, including the strandedness and directional asymmetry of the double helix, play a defining role in CT efficiency. For an (AT)n bridge, the orientation of the base pairs is critical; the yield of intrastrand HT is markedly higher through (A)n compared with (T)n bridges, whereas HT via intrastrand pathways is more efficient than through interstrand pathways. Remarkably, for reactions through the same DNA bridge, over the same distance, and with the same driving force, HT from photoexcited Ap to G in the 5' to 3' direction is more efficient and less dependent on distance than HT from 3' to 5'. We attribute these differences in HT efficiency to variations in base-base coupling within the DNA assemblies. Thus base-base coupling is a critical parameter in DNA CT and strongly depends on subtle structural nuances of duplex DNA.
Figures

References
-
- Dekker C. & Ratner, M. A. (2001) Phys. World 14, 29-33.
-
- Boon E. M. & Barton, J. K. (2002) Curr. Opin. Struct. Biol. 12, 320-329. - PubMed
-
- Giese B. (2002) Annu. Rev. Biochem. 71, 51-70. - PubMed
-
- Schuster G. B. (2000) Acc. Chem. Res. 33, 253-260. - PubMed
-
- Lewis F. D., Letsinger, R. L. & Wasielewski, M. R. (2001) Acc. Chem. Res. 34, 159-170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

