Topology of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum (RyR1)
- PMID: 12486242
- PMCID: PMC139211
- DOI: 10.1073/pnas.012688999
Topology of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum (RyR1)
Abstract
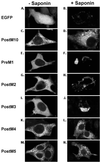
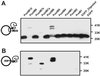
To define the topology of the skeletal muscle ryanodine receptor (RyR1), enhanced GFP (EGFP) was fused in-frame to the C terminus of RyR1, replacing a series of C-terminal deletions that started near the beginning or the end of predicted transmembrane helices M1-M10. The constructs were expressed in HEK-293 (human embryonic kidney cell line 293) or mouse embryonic fibroblast (MEF) cells, and confocal microscopy of intact and saponin-permeabilized cells was used to determine the subcellular location of the truncated fusion proteins. The fusion protein truncated after M3 exhibited uniform cytoplasmic fluorescence, which was lost after permeabilization, indicating that proposed M', M", M1, M2, and M3 sequences are not membrane-associated. The fusion protein truncated at the end of the M4-M5 loop and containing M4 was membrane-associated. All longer truncated fusion proteins were also associated with intracellular membranes. Mapping by protease digestion and extraction of isolated microsomes demonstrated that EGFP positioned after either M5, the N-terminal half of M7 (M7a), or M8 was located in the lumen, and that EGFP positioned after either M4, M6, the C-terminal half of M7 (M7b), or M10 was located in the cytoplasm. These results indicate that RyR1 contains eight transmembrane helices, organized as four hairpin loops. The first hairpin is likely to be made up of M4a-M4b. However, it could be made up from M3-M4, which might form a hairpin loop even though M3 alone is not membrane-associated. The other three hairpin loops are formed from M5-M6, M7a-M7b, and M8-M10. M9 is not a transmembrane helix, but it might form a selectivity filter between M8 and M10.
Figures


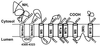
Similar articles
-
Role of the sequence surrounding predicted transmembrane helix M4 in membrane association and function of the Ca(2+) release channel of skeletal muscle sarcoplasmic reticulum (ryanodine receptor isoform 1).J Biol Chem. 2004 Sep 3;279(36):37566-74. doi: 10.1074/jbc.M406637200. Epub 2004 Jun 28. J Biol Chem. 2004. PMID: 15226293
-
Structure and targeting of RyR1: implications from fusion of green fluorescent protein at the amino-terminal.Arch Biochem Biophys. 2001 Apr 1;388(1):13-7. doi: 10.1006/abbi.2000.2263. Arch Biochem Biophys. 2001. PMID: 11361129
-
Does lens intrinsic membrane protein MP19 contain a membrane-targeting signal?Mol Vis. 2003 Dec 22;9:735-46. Mol Vis. 2003. PMID: 14735063
-
Membrane topology and membrane retention of the ryanodine receptor calcium release channel.Cell Biochem Biophys. 2004;40(2):207-24. doi: 10.1385/CBB:40:2:207. Cell Biochem Biophys. 2004. PMID: 15054223 Review.
-
Control of muscle ryanodine receptor calcium release channels by proteins in the sarcoplasmic reticulum lumen.Clin Exp Pharmacol Physiol. 2009 Mar;36(3):340-5. doi: 10.1111/j.1440-1681.2008.05094.x. Clin Exp Pharmacol Physiol. 2009. PMID: 19278523 Review.
Cited by
-
Identification of ATP-binding regions in the RyR1 Ca²⁺ release channel.PLoS One. 2012;7(11):e48725. doi: 10.1371/journal.pone.0048725. Epub 2012 Nov 7. PLoS One. 2012. PMID: 23144945 Free PMC article.
-
Building Biological Relevance Into Integrative Modelling of Macromolecular Assemblies.Front Mol Biosci. 2022 Apr 11;9:826136. doi: 10.3389/fmolb.2022.826136. eCollection 2022. Front Mol Biosci. 2022. PMID: 35480882 Free PMC article.
-
Coordinated movement of cytoplasmic and transmembrane domains of RyR1 upon gating.PLoS Biol. 2009 Apr 14;7(4):e85. doi: 10.1371/journal.pbio.1000085. PLoS Biol. 2009. PMID: 19402748 Free PMC article.
-
Central core disease and susceptibility to malignant hyperthermia in a single family.J Neurol. 2009 Jul;256(7):1161-3. doi: 10.1007/s00415-009-5051-4. Epub 2009 Feb 28. J Neurol. 2009. PMID: 19252784 No abstract available.
-
Architecture and conformational switch mechanism of the ryanodine receptor.Nature. 2015 Jan 1;517(7532):39-43. doi: 10.1038/nature13916. Epub 2014 Dec 1. Nature. 2015. PMID: 25470059
References
-
- Fleischer S. & Inui, M. (1989) Annu. Rev. Biophys. Biophys. Chem. 18, 333-364. - PubMed
-
- Franzini-Armstrong C. & Protasi, F. (1997) Physiol. Rev. 77, 699-729. - PubMed
-
- Lai F. A., Misra, M., Xu, L., Smith, H. A. & Meissner, G. (1989) J. Biol. Chem. 264, 16776-16785. - PubMed
-
- Takeshima H., Nishimura, S., Matsumoto, T., Ishida, H., Kangawa, K., Minamino, N., Matsuo, H., Ueda, M., Hanaoka, M., Hirose, T. & Numa, S. (1989) Nature 339, 439-445. - PubMed
-
- Zorzato F., Fujii, J., Otsu, K., Phillips, M., Green, N. M., Lai, F. A., Meissner, G. & MacLennan, D. H. (1990) J. Biol. Chem. 265, 2244-2256. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

