A randomised, double masked, multicentre clinical trial comparing bimatoprost and timolol for the treatment of glaucoma and ocular hypertension
- PMID: 12488264
- PMCID: PMC1771463
- DOI: 10.1136/bjo.87.1.57
A randomised, double masked, multicentre clinical trial comparing bimatoprost and timolol for the treatment of glaucoma and ocular hypertension
Abstract
Aim: To evaluate the safety and efficacy of bimatoprost 0.03% once daily or twice daily compared with timolol 0.5% twice daily in patients with glaucoma or ocular hypertension.
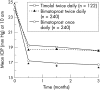
Methods: Multicentre, double masked, randomised, parallel group, 3 month trial comparing bimatoprost once daily (n=240), bimatoprost twice daily (n=240), and timolol twice daily (n=122). The primary efficacy end point was diurnal intraocular pressure (IOP) (8 am, 10 am, 4 pm). Safety measures included adverse events, ocular parameters, and systemic variables.
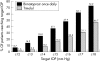
Results: Bimatoprost once daily provided significantly lower mean IOP than timolol twice daily at all times and follow up visits (p<0.001). At month 3, mean IOP reductions from baseline at 10 am (peak timolol effect) were bimatoprost once daily, 8.0 mm Hg (32.4%); bimatoprost twice daily, 6.3 mm Hg (25.2%); timolol, 5.5 mm Hg (22.7%). Bimatoprost twice daily was also more effective than timolol, but was not as effective as bimatoprost once daily. A higher percentage of patients achieved low target pressures with bimatoprost once daily than with timolol. The most frequent side effects with bimatoprost were eyelash growth and mild conjunctival hyperaemia. Systemic safety parameters were not affected by bimatoprost.
Conclusions: Bimatoprost 0.03% once daily demonstrated superior efficacy compared with timolol 0.5% twice daily in patients with elevated IOP. Bimatoprost once daily was more effective than twice daily dosing.
Figures

References
-
- Woodward DF, Tang-Liu DD-S, Madhu C, et al. Prostaglandin F2α (PGF2α) 1-ethanolamide: a pharmacologically unique local hormone biosynthesized from anandamide. 11th International Conference on Advances in Prostaglandin and Leukotriene Research, 2000.
-
- Krauss AH-P, Chen J, Protzman C, et al. Pharmacology, biosynthesis and ocular hypotensive activity of prostamide F2α (prostaglandin F2α 1-ethanoloamide), a novel naturally occurring substance. Association for Ocular Pharmacology and Therapeutics, 2000.
-
- Yu M, Ives D, Ramesha CS. Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J Biol Chem 1997;272:21181–6. - PubMed
-
- Woodward DF, Krauss AH-P, Chen J, et al. The pharmacology of bimatoprost (Lumigan). Surv Ophthalmol 2001;45(Suppl 4):S337–45. - PubMed
-
- Laibovitz RA, VanDenburgh AM, Felix C, et al. Comparison the ocular hypotensive lipid AGN 192024 with timolol: dosing, efficacy, and safety evaluation of a novel compound for glaucoma management. Arch Ophthalmol 2001;119:994–1000. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
