Clinical experience of e-PTFE membrane implant surgery for refractory glaucoma
- PMID: 12488265
- PMCID: PMC1771470
- DOI: 10.1136/bjo.87.1.63
Clinical experience of e-PTFE membrane implant surgery for refractory glaucoma
Abstract
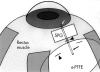
Aims: To evaluate the clinical efficacy of membrane tube implant made of expanded polytetrafluoroethylene (e-PTFE, Gore-Tex) membrane and silicone tube in treating refractory glaucoma.
Methods: A retrospective chart review was performed on 43 eyes of 40 patients who underwent glaucoma tube shunt implant surgery using double layered e-PTFE membrane and silicone tube to treat refractory glaucoma. The surgeries were performed from May 1991 to September 1995, and the subjects were patients with terminal glaucoma without useful vision on the study eye.
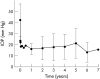
Results: The mean follow up period was 32.9 months. The Kaplan-Meier survival for intraocular pressure (IOP) control (IOP between 6 and 21mm Hg without significant complication) was 80.9% at 1 year, 73.9% at 2 years, and 62.2% at 3 years after surgery. After excluding three eyes of three patients who were dropped within 3 months after surgery and did not have any serious complication or problem in IOP control, the average preoperative IOP was 42.5 (SD 14.6) mm Hg and IOP on the last visit was 17.3 (10.2) mm Hg (p = 0.000, n = 40). The number of antiglaucoma medications before surgery (2.2 (0.6)) was reduced to 0.5 (0.8) on the last visit (p = 0.000). The IOP was controlled within the range of 6-21 mm Hg in 26 eyes (65.0%). In the remaining 14 eyes (35%), we could not control the IOP or additional surgery was needed to control the IOP or to treat severe complications. Two cases of endophthalmitis and three of phthisis were found as serious complications. The other complications were similar to those of other commercially available glaucoma implants.
Conclusion: A comparable clinical result was obtained with this new implant as with the other commercially available implants. This implant with a thin and non-rigid reservoir has a potential to reduce some complications associated with the large volume and rigid consistency of the other implants, although it is not yet proved. This membrane tube implant may be considered as another substitute in the surgery of refractory glaucoma.
Figures



References
-
- Williams AS. Setons in glaucoma surgery. In: Albert DM, Jakobiec FA, eds. Principles and practice of opthalmology. Philadelphia: WB Saunders, 1994:1655–67.
-
- Rosenberg LF, Krupin T. Implants in glaucoma surgery. In: Ritch R, Shields MB, Krupin T, ed. The glaucomas. 2nd ed. Philadelphia: Mosby, 1996:1783–807.
-
- Mills RP, Reynolds A, Emond MJ, et al. Long-term survival of Molteno Glaucoma Drainage Device. Ophthalmology 1996;103:299–305. - PubMed
-
- Coleman AL, Hill R, Wilson MR, et al. Initial clinical experience with the Ahmed glaucoma valve implant. Am J Ophthalmol 1995;120:23–31. - PubMed
-
- Siegner SW, Netland PA, Urban Jr RC, et al. Clinical experience with the Baerveldt Glaucoma Drainage Implant. Ophthalmology 1995;102:1298–307. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
