Multiepitope CD8(+) T cell response to a NY-ESO-1 peptide vaccine results in imprecise tumor targeting
- PMID: 12488431
- PMCID: PMC151653
- DOI: 10.1172/JCI16428
Multiepitope CD8(+) T cell response to a NY-ESO-1 peptide vaccine results in imprecise tumor targeting
Abstract
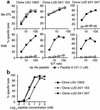
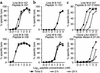
The cancer-testis antigen NY-ESO-1 is one of the most promising candidates for generic vaccination of cancer patients. Here we analyzed the CD8(+) T cell response to a NY-ESO-1 peptide vaccine composed of the two previously defined peptides 157-165 and 157-167, administered with GM-CSF as a systemic adjuvant. The NY-ESO-1 peptide vaccine elicited a CD8(+) T cell response directed against multiple distinct epitopes in the 157-167 region, as revealed by using A2/peptide multimers incorporating overlapping A2 binding peptides in this region. However, only a minor fraction of the elicited CD8(+) T cells, namely those recognizing the peptide 157-165 with sufficiently high functional avidity, recognized the naturally processed target on NY-ESO-1(+) tumor cells. In contrast, the majority of peptide 157-165-specific CD8(+) T cells exhibited lower functional avidity and no tumor reactivity. In addition, vaccine-elicited CD8(+) T cells specific for other overlapping epitopes in the 157-167 region failed to significantly recognize NY-ESO-1-expressing tumor targets. Thus, because of the complexity of the CD8(+) T cell repertoire that can be elicited by vaccination with synthetic peptides, a precise definition of the targeted epitope, and hence, of the corresponding peptide to be used as immunogen, is required to ensure a precise tumor targeting.
Figures




Comment on
-
Getting peptide vaccines to work: just a matter of quality control?J Clin Invest. 2002 Dec;110(12):1765-8. doi: 10.1172/JCI17405. J Clin Invest. 2002. PMID: 12488425 Free PMC article. No abstract available.
References
-
- Labrecque S, Naor N, Thomson D, Matlashewski G. Analysis of the anti-p53 antibody response in cancer patients. Cancer Res. 1993;53:3468–3471. - PubMed
-
- Cheever MA, et al. Immunity to oncogenic proteins. Immunol. Rev. 1995;145:33–59. - PubMed
-
- Tindle RW. Human papillomavirus vaccines for cervical cancer. Curr. Opin. Immunol. 1996;8:643–650. - PubMed
-
- van der Bruggen P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

