Characterization of mouse clonogenic megakaryocyte progenitors
- PMID: 12490656
- PMCID: PMC140928
- DOI: 10.1073/pnas.262655099
Characterization of mouse clonogenic megakaryocyte progenitors
Abstract
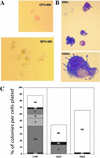
Although it has been shown that unfractionated bone marrow, hematopoietic stem cells, common myeloid progenitors, and bipotent megakaryocyteerythrocyte progenitors can give rise to megakaryocyte colonies in culture, monopotent megakaryocyte-committed progenitors (MKP) have never been prospectively isolated from the bone marrow of adult mice. Here, we use a monoclonal antibody to the megakaryocyte-associated surface protein, CD9, to purify MKPs from the c-kit(+)Sca-1(-)IL7Ralpha(-)Thy1.1(-)Lin(-) fraction of adult C57BLKa-Thy1.1 bone marrow. The CD9(+) fraction contained a subset of CD41(+)FcgammaR(lo)CD34(+)CD38(+) cells that represent approximately 0.01% of the total nucleated bone marrow cells. They give rise mainly to colony-forming unit-megakaryocytes and occasionally burst-forming unit-megakaryocytes, with a plating efficiency >60% at the single-cell level. In vivo, MKPs do not have spleen colony-forming activity nor do they contribute to long-term multilineage hematopoiesis; they give rise only to platelets for approximately 3 weeks. Common myeloid progenitors and megakaryocyteerythrocyte progenitors can differentiate into MKPs after 72 h in stromal cultures, indicating that MKPs are downstream of these two progenitors. These isolatable MKPs will be very useful for further studies of megakaryopoiesis as well as the elucidation of their gene expression patterns.
Figures



References
-
- Weissman I L, Anderson D J, Gage F. Annu Rev Cell Dev Biol. 2001;17:387–403. - PubMed
-
- Metcalf D. Ann NY Acad Sci. 1999;872:289–303. - PubMed
-
- Bradley T R, Metcalf D. Aust J Exp Biol Med Sci. 1966;44:287–299. - PubMed
-
- Eaves C J. In: Williams Hematology. Beutler E, Lichtman M A, Coller B S, Kipps T J, editors. New York: McGraw–Hill; 1995. pp. L22–L26.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

