Propynyl groups in duplex DNA: stability of base pairs incorporating 7-substituted 8-aza-7-deazapurines or 5-substituted pyrimidines
- PMID: 12490717
- PMCID: PMC140073
- DOI: 10.1093/nar/gkf689
Propynyl groups in duplex DNA: stability of base pairs incorporating 7-substituted 8-aza-7-deazapurines or 5-substituted pyrimidines
Abstract
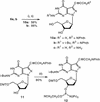
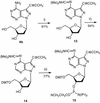
Oligonucleotides incorporating the 7-propynyl derivatives of 8-aza-7-deaza-2'-deoxyguanosine (3b) and 8-aza-7-deaza-2'-deoxyadenosine (4b) were synthesized and their duplex stability was compared with those containing the 5-propynyl derivatives of 2'-deoxycytidine (1) and 2'-deoxyuridine (2). For this purpose phosphoramidites of the 8-aza- 7-deazapurine (pyrazolo[3,4-d]pyrimidine) nucleosides were prepared and employed in solid-phase synthesis. All propynyl nucleosides exert a positive effect on the DNA duplex stability because of the increased polarizability of the nucleobase and the hydrophobic character of the propynyl group. The propynyl residues introduced into the 7-position of the 8-aza-7-deazapurines are generally more stabilizing than those at the 5-position of the pyrimidine bases. The duplex stabilization of the propynyl derivative 4b was higher than for the bromo nucleoside 4c. The extraordinary stability of duplexes containing the 7-propynyl derivative of 8-aza-7- deazapurin-2,6-diamine (5b) is attributed to the formation of a third hydrogen bond, which is apparently not present in the base pair of the purin-2,6-diamine 2'-deoxyribonucleoside with dT.
Figures












Similar articles
-
Oligonucleotides incorporating 8-aza-7-deazapurines: synthesis and base pairing of nucleosides with nitrogen-8 as a glycosylation position.Org Biomol Chem. 2003 Jun 7;1(11):1873-83. doi: 10.1039/b301608k. Org Biomol Chem. 2003. PMID: 12945768
-
Pyrazolo[3,4-d]pyrimidine nucleic acids: adjustment of dA-dT to dG-dC base pair stability.Nucleic Acids Res. 2001 May 15;29(10):2069-78. doi: 10.1093/nar/29.10.2069. Nucleic Acids Res. 2001. PMID: 11353076 Free PMC article.
-
Propynyl groups in duplex, hairpin and triplex DNA: 7-deazapurines and 9-deazapurines.Nucleosides Nucleotides Nucleic Acids. 2005;24(5-7):851-4. doi: 10.1081/ncn-200059184. Nucleosides Nucleotides Nucleic Acids. 2005. PMID: 16248048
-
Stabilization of tandem dG-dA base pairs in DNA-hairpins: replacement of the canonical bases by 7-deaza-7-propynylpurines.Org Biomol Chem. 2005 Dec 7;3(23):4221-6. doi: 10.1039/b510444k. Epub 2005 Oct 20. Org Biomol Chem. 2005. PMID: 16294250
-
Progress in 7-deazapurine - pyrrolo[2,3-d]pyrimidine - ribonucleoside synthesis.Curr Top Med Chem. 2006;6(9):867-92. doi: 10.2174/156802606777303649. Curr Top Med Chem. 2006. PMID: 16787281 Review.
Cited by
-
Reactive Acrylamide-Modified DNA Traps for Accurate Cross-Linking with Cysteine Residues in DNA-Protein Complexes Using Mismatch Repair Protein MutS as a Model.Molecules. 2022 Apr 10;27(8):2438. doi: 10.3390/molecules27082438. Molecules. 2022. PMID: 35458636 Free PMC article.
-
Enzymatic synthesis of hypermodified DNA polymers for sequence-specific display of four different hydrophobic groups.Nucleic Acids Res. 2020 Dec 2;48(21):11982-11993. doi: 10.1093/nar/gkaa999. Nucleic Acids Res. 2020. PMID: 33152081 Free PMC article.
-
Nuclear magnetic resonance reveals a two hairpin equilibrium near the 3'-splice site of influenza A segment 7 mRNA that can be shifted by oligonucleotides.RNA. 2022 Apr;28(4):508-522. doi: 10.1261/rna.078951.121. Epub 2022 Jan 4. RNA. 2022. PMID: 34983822 Free PMC article.
-
De novo DNA synthesis using polymerase-nucleotide conjugates.Nat Biotechnol. 2018 Aug;36(7):645-650. doi: 10.1038/nbt.4173. Epub 2018 Jun 18. Nat Biotechnol. 2018. PMID: 29912208
-
Enzymatic primer-extension with glycerol-nucleoside triphosphates on DNA templates.PLoS One. 2009;4(3):e4949. doi: 10.1371/journal.pone.0004949. Epub 2009 Mar 23. PLoS One. 2009. PMID: 19305495 Free PMC article.
References
-
- Seela F. and Becher,G. (1998) Synthesis of 7-halogenated 8-aza-7-deaza-2′-deoxy-guanosines and related pyrazolo[3,4-d]pyrimidine 2′-deoxyribonucleosides. Synthesis, 207–214.
-
- Seela F. and Steker,H. (1986) Synthesis of 2′-deoxyribofuranosides of 8-aza-7-deazaguanine and related pyrazolo[3,4-d]pyrimidines. Helv. Chim. Acta, 69, 1602–1613.
-
- Seela F., Winkeler,H.-D., Driller,H. and Menkhoff,S. (1984) Synthesis of 7-deaza-2′-deoxyguanosine by phase-transfer glycosylation and preparation of suitable derivatives for oligonucleotide synthesis. Nucleic Acids Res., 14, 245–246.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

