Specificity of Mnt 'master residue' obtained from in vivo and in vitro selections
- PMID: 12490722
- PMCID: PMC140065
- DOI: 10.1093/nar/gkf684
Specificity of Mnt 'master residue' obtained from in vivo and in vitro selections
Abstract
Mnt is a repressor from phage P22 that belongs to the ribbon-helix-helix family of DNA binding factors. Four amino acids from the N-terminus of the protein, Arg2, His6, Asn8 and Arg10, interact with the base pairs of the DNA to provide the sequence specificity. Raumann et al. (Nature Struct. Biol., 2, 1115-1122) identified position 6 as a 'master residue' that controls the specificity of the protein. Models for the interaction have residue 6 of Mnt interacting directly with position 5 of the operator. In vivo selections demonstrated that protein variants at residue 6 bound specifically to operator mutations at that position. Operators in which the wild-type G at position 5 was replaced by T specifically bound to several different protein variants, primarily hydrophobic residues. The obtained protein variants, plus some others, were used in in vitro selections to determine their preferred binding sites. The results showed that the residue at position 6 influenced the preference for binding site bases predominantly at position 5, but that the effects of altering it can extend over longer distances, consistent with its designation as a 'master residue'. The similarities of binding sites for different residues do not correlate strongly with common measures of amino acid similarities.
Figures
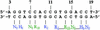
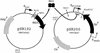

References
-
- Vershon A.K., Youderian,P., Susskind,M.M. and Sauer,R.T. (1985) The Bacteriophage P22 Arc and Mnt repressors. J. Biol. Chem., 260, 12124–12129. - PubMed
-
- Vershon K.A., Liao,S.-M., McClure,W.R. and Sauer,R.T. (1987) Bacteriophage P22 Mnt repressor DNA binding and effects on transcription in vitro. J. Mol. Biol., 195, 311–322. - PubMed
-
- Knight K.L. and Sauer,R.T.(1989b) Identification of functionally important residues in the DNA binding region of Mnt repressor. J. Biol. Chem., 264, 13706–13710. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

