The organizing principle: microenvironmental influences in the normal and malignant breast
- PMID: 12492495
- PMCID: PMC2933198
- DOI: 10.1046/j.1432-0436.2002.700907.x
The organizing principle: microenvironmental influences in the normal and malignant breast
Abstract
The current paradigm for cancer initiation and progression rests on the groundbreaking discoveries of oncogenes and tumor suppressor genes. This framework has revealed much about the role of genetic alterations in the underlying signaling pathways central to normal cellular function and to tumor progression. However, it is clear that single gene theories or even sequential acquisition of mutations underestimate the nature of the genetic and epigenetic changes in tumors, and do not account for the observation that many cancer susceptibility genes (e.g. BRCA1, APC) show a high degree of tissue specificity in their association with neoplastic transformation. Therefore, the cellular and tissue context itself must confer additional and crucial information necessary for mutated genes to exert their influence. A considerable body of evidence now shows that cell-cell and cell-extracellular matrix (ECM) interactions are essential organizing principles that help define the nature of the tissue context, and play a crucial role in regulating homeostasis and tissue specificity. How this context determines functional integrity, and how its loss can lead to malignancy, appears to have much to do with tissue structure and polarity.
Figures



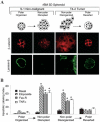
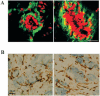
References
-
- Airola K, Fusenig NE. Differential stromal regulation of MMP-1 expression in benign and malignant keratinocytes. J Invest Dermatol. 2001;116:85–92. - PubMed
-
- Bissell MJ. The differentiated state of normal and malignant cells or how to define a “normal” cell in culture. Int Rev Cytol. 1981;70:27–100. - PubMed
-
- Bissell MJ, Weaver VM, Lelievre SA, Wang F, Petersen OW, Schmeichel KL. Tissue structure, nuclear organization, and gene expression in normal and malignant breast. Cancer Res. 1999;59:1757s–1763s. discussion 1763s–1764s. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

