The CD1 family and T cell recognition of lipid antigens
- PMID: 12492810
- PMCID: PMC7182298
- DOI: 10.1034/j.1399-0039.2002.600501.x
The CD1 family and T cell recognition of lipid antigens
Abstract
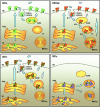
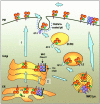
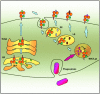
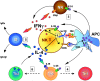
For many years it was thought that T lymphocytes recognized only peptide antigens presented by MHC class I or class II molecules. Recently, it has become clear that a wide variety of lipids and glycolipids are also targets of the T cell response. This novel form of cell-mediated immune recognition is mediated by a family of lipid binding and presenting molecules known as CD1. The CD1 proteins represent a small to moderate sized family of beta2-microglobulin-associated transmembrane proteins that are distantly related to MHC class I and class II molecules. They are conserved in most or all mammals, and control the development and function of T cell populations that participate in innate and adaptive immune responses through the recognition of self and foreign lipid antigens. Here we review the current state of our understanding of the structure and function of CD1 proteins, and the role of CD1-restricted T cell responses in the immune system.
Figures

Similar articles
-
CD1 proteins: targets of T cell recognition in innate and adaptive immunity.Rev Immunogenet. 2000;2(3):416-32. Rev Immunogenet. 2000. PMID: 11256748 Review.
-
The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids.Annu Rev Immunol. 1999;17:297-329. doi: 10.1146/annurev.immunol.17.1.297. Annu Rev Immunol. 1999. PMID: 10358761 Review.
-
Mechanisms of lipid antigen presentation by CD1.Crit Rev Immunol. 1999;19(1):49-63. Crit Rev Immunol. 1999. PMID: 9987600 Review.
-
CD1 antigen presentation: how it works.Nat Rev Immunol. 2007 Dec;7(12):929-41. doi: 10.1038/nri2191. Nat Rev Immunol. 2007. PMID: 18037897 Review.
-
Antigen presentation by CD1 molecules and the generation of lipid-specific T cell immunity.Cell Mol Life Sci. 2007 Jul;64(14):1824-40. doi: 10.1007/s00018-007-7007-0. Cell Mol Life Sci. 2007. PMID: 17483872 Free PMC article. Review.
Cited by
-
Evasion and subversion of antigen presentation by Mycobacterium tuberculosis.Tissue Antigens. 2009 Sep;74(3):189-204. doi: 10.1111/j.1399-0039.2009.01301.x. Epub 2009 Jun 25. Tissue Antigens. 2009. PMID: 19563525 Free PMC article. Review.
-
Leishmania infantum amastigotes trigger a subpopulation of human B cells with an immunoregulatory phenotype.PLoS Negl Trop Dis. 2015 Feb 24;9(2):e0003543. doi: 10.1371/journal.pntd.0003543. eCollection 2015 Feb. PLoS Negl Trop Dis. 2015. PMID: 25710789 Free PMC article.
-
Mycobacteria and other environmental organisms as immunomodulators for immunoregulatory disorders.Springer Semin Immunopathol. 2004 Feb;25(3-4):237-55. doi: 10.1007/s00281-003-0148-9. Epub 2003 Oct 8. Springer Semin Immunopathol. 2004. PMID: 15007629 Review.
-
TLR-independent induction of human monocyte IL-1 by phosphoglycolipids from thermophilic bacteria.Glycoconj J. 2008 Jul;25(5):427-39. doi: 10.1007/s10719-007-9088-2. Epub 2007 Dec 27. Glycoconj J. 2008. PMID: 18161025
-
Mycobacterium tuberculosis controls host innate immune activation through cyclopropane modification of a glycolipid effector molecule.J Exp Med. 2005 Feb 21;201(4):535-43. doi: 10.1084/jem.20041668. Epub 2005 Feb 14. J Exp Med. 2005. PMID: 15710652 Free PMC article.
References
-
- Porcelli SA, Modlin RL. The CD1 system: antigen‐presenting molecules for T cell recognition of lipids and glycolipids. Annu Rev Immunol 1999: 17: 297–329. - PubMed
-
- Calabi F, Milstein C. The molecular biology of CD1. Semin Immunol 2000: 12: 503–9. - PubMed
-
- Calabi F, Jarvis JM, Martin L, Milstein C. Two classes of CD1 genes. Eur J Immunol 1989: 19: 285–92. - PubMed
-
- Angenieux C, Salamero J, Fricker D et al. Characterization of CD1e, a third type of CD1 molecule expressed in dendritic cells. J Biol Chem 2000: 275: 37757–64. - PubMed
-
- Brossay L, Jullien D, Cardell S et al. Mouse CD1 is mainly expressed on hemopoietic‐derived cells. J Immunol 1997: 159: 1216–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

