Interaction of pulmonary surfactant protein C with CD14 and lipopolysaccharide
- PMID: 12496149
- PMCID: PMC143282
- DOI: 10.1128/IAI.71.1.61-67.2003
Interaction of pulmonary surfactant protein C with CD14 and lipopolysaccharide
Abstract
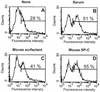
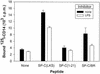
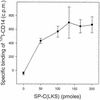
In addition to their effects on alveolar surface tension, some components of lung surfactant also have immunological functions. We found recently that the hydrophobic lung surfactant protein SP-C specifically binds to the lipid A region of lipopolysaccharide (LPS). In this study, we show that SP-C also interacts with CD14. Four observations showed cross talk between the three molecules SP-C, LPS, and CD14. (i) Like LBP, SP-C allows the binding of a fluorescent LPS to cells expressing CD14 (the other surfactant components were ineffective). (ii) Recombinant radiolabeled CD14 and SP-C (or a synthetic analog of SP-C) interact in a dose-dependent manner. (iii) LPS blocks the binding of radiolabeled CD14 to SP-C-coated wells. (iv) SP-C enhances the binding of radiolabeled CD14 to LPS-coated wells. These results, obtained with native murine SP-C and with three synthetic analogs, suggest that LPS and CD14 interact with the same region of SP-C and that binding of SP-C modifies the conformation of CD14 or the accessibility of its LPS-binding site, allowing it to bind LPS. This ability of SP-C to interact with the pattern recognition molecule CD14 extends the possible immunological targets of SP-C to a large panel of microorganisms that can enter the airways.
Figures




References
-
- Akashi, S., R. Shimazu, H. Ogata, Y. Nagai, K. Takeda, M. Kimoto, and K. Miyake. 2000. Cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J. Immunol. 164:3471-3475. - PubMed
-
- Augusto, L., K. Le Blay, G. Auger, D. Blanot, and R. Chaby. 2001. Interaction of bacterial lipopolysaccharide with mouse surfactant protein C inserted into lipid vesicles. Am. J. Physiol. Lung Cell. Mol. Physiol. 281:L776-L785. - PubMed
-
- Augusto, L. A., J. Li, M. Synguelakis, J. Johansson, and R. Chaby. 2002. Structural basis for interactions between lung surfactant protein C and bacterial lipopolysaccharide. J. Biol. Chem. 277:23484-23492. - PubMed
-
- Baveye, S., E. Elass, D. G. Fernig, C. Blanquart, J. Mazurier, and D. Legrand. 2000. Human lactoferrin interacts with soluble CD14 and inhibits expression of endothelial adhesion molecules, E-selectin and ICAM-1, induced by the CD14-lipopolysaccharide complex. Infect. Immun. 68:6519-6525. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

