Divergent patterns of colonization and immune response elicited from two intestinal Lactobacillus strains that display similar properties in vitro
- PMID: 12496193
- PMCID: PMC143181
- DOI: 10.1128/IAI.71.1.428-436.2003
Divergent patterns of colonization and immune response elicited from two intestinal Lactobacillus strains that display similar properties in vitro
Abstract
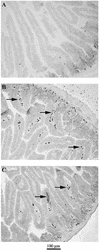
Lactobacilli derived from the endogenous flora of normal donors are being increasingly used as probiotics in functional foods and as vaccine carriers. However, a variety of studies done with distinct strains of lactobacilli has suggested heterogeneous and strain-specific effects. To dissect this heterogeneity at the immunological level, we selected two strains of lactobacilli that displayed similar properties in vitro and studied their impact on mucosal and systemic B-cell responses in monoxenic mice. Germfree mice were colonized with Lactobacillus johnsonii (NCC 533) or Lactobacillus paracasei (NCC 2461). Bacterial loads were monitored for 30 days in intestinal tissues, and mucosal and systemic B-cell responses were measured. Although both Lactobacillus strains displayed similar growth, survival, and adherence properties in vitro, they colonized the intestinal lumen and translocated into mucosal lymphoid organs at different densities. L. johnsonii colonized the intestine very efficiently at high levels, whereas the number of L. paracasei decreased rapidly and it colonized at low levels. We determined whether this difference in colonization correlated with an induction of different types of immune responses. We observed that colonization with either strain induced similar germinal center formation and immunoglobulin A-bearing lymphocytes in the mucosa, suggesting that both strains were able to activate mucosal B-cell responses. However, clear differences in patterns of immunoglobulins were observed between the two strains in the mucosa and in the periphery. Therefore, despite similar in vitro probiotic properties, distinct Lactobacillus strains may colonize the gut differently and generate divergent immune responses.
Figures



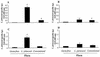

References
-
- Albanese, C. T., S. D. Smith, S. Watkins, A. Kurkchubasche, R. L. Simmons, and M. I. Rowe. 1994. Effect of secretory IgA on transepithelial passage of bacteria across the intact ileum in vitro. J. Am. Coll. Surg. 179:679-688. - PubMed
-
- Ayabe, T., D. P. Satchell, C. L. Wilson, W. C. Parks, M. E. Selsted, and A. J. Ouellette. 2000. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 1:113-118. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

