Contribution of GIRK2-mediated postsynaptic signaling to opiate and alpha 2-adrenergic analgesia and analgesic sex differences
- PMID: 12496346
- PMCID: PMC140949
- DOI: 10.1073/pnas.0136822100
Contribution of GIRK2-mediated postsynaptic signaling to opiate and alpha 2-adrenergic analgesia and analgesic sex differences
Abstract
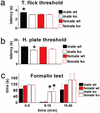
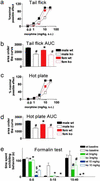
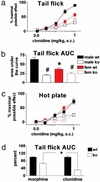
The analgesia produced by inhibitory G protein-coupled receptor agonists involves coordinated postsynaptic inhibition via G protein-coupled inwardly rectifying potassium channels (GIRKs) and presynaptic inhibition of neurotransmitter release through regulation of voltage-gated Ca(2+) channels. Here, we used mice lacking the GIRK2 channel subunit to assess the relative contribution of these two effector systems to nociceptive processing in male and female mice. Compared with female WT mice, male WT mice exhibited higher pain thresholds and enhanced opioid (morphine) and alpha(2)-adrenergic (clonidine) receptor-induced antinociception in a spinal reflex test. The GIRK2-null mutation reduced the "pain" threshold in male but not in female mice, effectively eliminating the sex differences in pain threshold. In addition, deletion of GIRK2 channels in mutant mice largely eliminated clonidine antinociception and significantly decreased morphine antinociception. Furthermore, the more pronounced morphine and clonidine-induced antinociception in male mice disappeared in the GIRK2 mutants. Based on the almost complete loss of clonidine-induced antinociception in the mutant mice, we conclude that it is primarily mediated by postsynaptic alpha(2)-adrenergic receptors. In contrast, the significant residual morphine effect in the mutant mice points to the presynaptic mu opioid receptor as a major contributor to its analgesic action. Finally, our results suggest that the reduced pain responsiveness of male compared with female mice results in part from GIRK2-coupled postsynaptic receptors that are activated by endogenous antinociceptive systems.
Figures
Similar articles
-
A pervasive mechanism for analgesia: activation of GIRK2 channels.Proc Natl Acad Sci U S A. 2003 Jan 7;100(1):277-82. doi: 10.1073/pnas.012682399. Epub 2002 Dec 19. Proc Natl Acad Sci U S A. 2003. PMID: 12493843 Free PMC article.
-
Spinal G-protein-gated K+ channels formed by GIRK1 and GIRK2 subunits modulate thermal nociception and contribute to morphine analgesia.J Neurosci. 2004 Mar 17;24(11):2806-12. doi: 10.1523/JNEUROSCI.5251-03.2004. J Neurosci. 2004. PMID: 15028774 Free PMC article.
-
Spinal G-protein-gated potassium channels contribute in a dose-dependent manner to the analgesic effect of mu- and delta- but not kappa-opioids.J Neurosci. 2005 Apr 6;25(14):3551-9. doi: 10.1523/JNEUROSCI.4899-04.2005. J Neurosci. 2005. PMID: 15814785 Free PMC article.
-
Molecular mechanisms of analgesia induced by opioids and ethanol: is the GIRK channel one of the keys?Neurosci Res. 2002 Oct;44(2):121-131. doi: 10.1016/s0168-0102(02)00094-9. Neurosci Res. 2002. PMID: 12354627 Review.
-
Pharmacological profiles of alpha 2 adrenergic receptor agonists identified using genetically altered mice and isobolographic analysis.Pharmacol Ther. 2009 Aug;123(2):224-38. doi: 10.1016/j.pharmthera.2009.04.001. Epub 2009 Apr 23. Pharmacol Ther. 2009. PMID: 19393691 Free PMC article. Review.
Cited by
-
Identification of a G-Protein-Independent Activator of GIRK Channels.Cell Rep. 2020 Jun 16;31(11):107770. doi: 10.1016/j.celrep.2020.107770. Cell Rep. 2020. PMID: 32553165 Free PMC article.
-
Attenuated G protein signaling and minimal receptor phosphorylation as a biochemical signature of low side-effect opioid analgesics.Sci Rep. 2022 May 3;12(1):7154. doi: 10.1038/s41598-022-11189-6. Sci Rep. 2022. PMID: 35504962 Free PMC article.
-
Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease.Nat Rev Neurosci. 2010 May;11(5):301-15. doi: 10.1038/nrn2834. Epub 2010 Apr 14. Nat Rev Neurosci. 2010. PMID: 20389305 Free PMC article. Review.
-
Ferroptosis inhibitor ferrostatin-1 attenuates morphine tolerance development in male rats by inhibiting dorsal root ganglion neuronal ferroptosis.Korean J Pain. 2024 Jul 1;37(3):233-246. doi: 10.3344/kjp.24042. Korean J Pain. 2024. PMID: 38946696 Free PMC article.
-
Pharmacogenetics of Addiction Therapy.Methods Mol Biol. 2022;2547:437-490. doi: 10.1007/978-1-0716-2573-6_16. Methods Mol Biol. 2022. PMID: 36068473
References
-
- Rollman G B, Lautenbacher S, Jones K. In: Sex, Gender, and Pain. Filingim R B, editor. Vol. 17. Seattle: International Association for the Study of Pain; 2000. pp. 165–190.
-
- Miaskowski C, Gear R W, Levine J D. In: Sex, Gender, and Pain. Filingim R B, editor. Vol. 17. Seattle: International Association for the Study of Pain; 2000. pp. 209–230.
-
- Sarton E, Olofsen E, Romberg R, den Hartigh J, Kest B, Nieuwenhuijs D, Burm A, Teppema L, Dahan A. Anesthesiology. 2000;93:1245–1254. - PubMed
-
- Kest B, Sarton E, Dahan A. Anesthesiology. 2000;93:539–547. - PubMed
-
- Sternberg W F, Wachterman M W. In: Sex, Gender, and Pain. Filingim R B, editor. Vol. 17. Seattle: International Association for the Study of Pain; 2000. pp. 71–88.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

