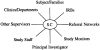
The invisible hand in clinical research: the study coordinator's critical role in human subjects protection
- PMID: 12497701
- PMCID: PMC4819323
- DOI: 10.1111/j.1748-720x.2002.tb00410.x
The invisible hand in clinical research: the study coordinator's critical role in human subjects protection
Figures
References
-
- Hovde M, Seskin R. Selecting U.S. Clinical Investigators. Applied Clinical Trials. 1997;8:34–42.
-
- Department of Health and Human Services, Office of Inspector General. The Globalization of Clinical Trials: A Growing Challenge in Protecting Human Subjects. 2001 Sep; OEI-01-00-00190. available at < http://oig.hhs.gov/oei/reports/oei-01-00-00190.pdf>. - PubMed
-
- Annas GJ. Reforming Informed Consent to Genetic Research. JAMA. 2001;286:2326–28. - PubMed
-
- King N, Henderson G, Stein J. Beyond Regulations: Ethics in Human Subjects Research. Chapel Hill: University of North Carolina Press; 1999.
-
- Papke A. The ACRP National Job Analysis of the Clinical Research Coordinator. The Monitor. 1996 Winter;:45–53. at 46.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical