Fourteen-member macrolides promote the phosphatidylserine receptor-dependent phagocytosis of apoptotic neutrophils by alveolar macrophages
- PMID: 12499168
- PMCID: PMC148990
- DOI: 10.1128/AAC.47.1.48-53.2003
Fourteen-member macrolides promote the phosphatidylserine receptor-dependent phagocytosis of apoptotic neutrophils by alveolar macrophages
Abstract
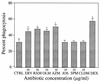
An inflammation of the airway of patients with diffuse panbronchiolitis (DPB), is characterized by dense neutrophil infiltration. Resolution of the inflammation can be achieved by the removal of apoptotic neutrophils by human alveolar macrophages (AM) without liberating neutrophil proteases in the airway. To understand clinical efficacy for the treatment of DPB by 14- or 15-member macrolides, their effects on the phagocytosis of apoptotic neutrophils by AM were examined. Treatment of AM with erythromycin (ERY) or clarithromycin at clinically achievable levels significantly increased the levels of phagocytosis of apoptotic neutrophils. A serum factor was not essential for the enhancement by these 14-member macrolides. Of the antibiotics tested, these effects were specific for the 14-member macrolides and a 15-member macrolide, azithromycin, but not for the 16-member macrolides, clindamycin or beta-lactam antibiotics. The enhanced phagocytosis of apoptotic neutrophils by ERY had no effect on the levels of interleukin-8 or tumor necrosis factor alpha production by lipopolysaccharide-stimulated AM after phagocytosis of the apoptotic neutrophils. The increased phagocytosis of apoptotic neutrophils by ERY was also found to be phosphatidylserine receptor-dependent for AM. These data indicate a novel anti-inflammatory action of 14-member and 15-member macrolides, and suggest that such antibiotics achieve clinical efficacy for patients with DPB, in part, through enhancing the nonphlogistic phagocytosis of apoptotic neutrophils by AM.
Figures
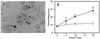
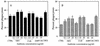
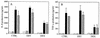
Similar articles
-
Nonantibiotic macrolides restore airway macrophage phagocytic function with potential anti-inflammatory effects in chronic lung diseases.Am J Physiol Lung Cell Mol Physiol. 2017 May 1;312(5):L678-L687. doi: 10.1152/ajplung.00518.2016. Epub 2017 Mar 3. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28258107 Free PMC article.
-
Azithromycin increases phagocytosis of apoptotic bronchial epithelial cells by alveolar macrophages.Eur Respir J. 2006 Sep;28(3):486-95. doi: 10.1183/09031936.06.00001506. Epub 2006 May 31. Eur Respir J. 2006. PMID: 16737992
-
Fourteen-member macrolides suppress interleukin-8 production but do not promote apoptosis of activated neutrophils.Antimicrob Agents Chemother. 2002 Apr;46(4):1101-4. doi: 10.1128/AAC.46.4.1101-1104.2002. Antimicrob Agents Chemother. 2002. PMID: 11897597 Free PMC article.
-
Clinical implications of the immunomodulatory effects of macrolides.Am J Med. 2004 Nov 8;117 Suppl 9A:5S-11S. doi: 10.1016/j.amjmed.2004.07.023. Am J Med. 2004. PMID: 15586558 Review.
-
Mode of action of long-term low-dose macrolide therapy for chronic sinusitis in the light of neutrophil recruitment.Curr Drug Targets Inflamm Allergy. 2002 Mar;1(1):117-26. doi: 10.2174/1568010023344832. Curr Drug Targets Inflamm Allergy. 2002. PMID: 14561211 Review.
Cited by
-
Macrolide immunomodulation of chronic respiratory diseases.Curr Infect Dis Rep. 2007 Jan;9(1):7-13. doi: 10.1007/s11908-007-0016-1. Curr Infect Dis Rep. 2007. PMID: 17254499
-
Efferocytosis in multisystem diseases (Review).Mol Med Rep. 2022 Jan;25(1):13. doi: 10.3892/mmr.2021.12529. Epub 2021 Nov 15. Mol Med Rep. 2022. PMID: 34779503 Free PMC article. Review.
-
Image-guided evaluation and monitoring of treatment response in patients with dry eye disease.Graefes Arch Clin Exp Ophthalmol. 2014 Jun;252(6):857-872. doi: 10.1007/s00417-014-2618-2. Epub 2014 Apr 4. Graefes Arch Clin Exp Ophthalmol. 2014. PMID: 24696045 Free PMC article. Review.
-
Phagocytosis, Degranulation and Extracellular Traps Release by Neutrophils-The Current Knowledge, Pharmacological Modulation and Future Prospects.Front Pharmacol. 2021 May 4;12:666732. doi: 10.3389/fphar.2021.666732. eCollection 2021. Front Pharmacol. 2021. PMID: 34017259 Free PMC article. Review.
-
In Vitro Anti-inflammatory and Immunomodulatory Effects of Ciprofloxacin or Azithromycin in Staphylococcus aureus-Stimulated Murine Macrophages are Beneficial in the Presence of Cytochalasin D.Inflammation. 2015;38(3):1050-69. doi: 10.1007/s10753-014-0070-4. Inflammation. 2015. PMID: 25429758
References
-
- Abe, S., H. Nakamura, S. Inoue, H. Takeda, H. Saito, S. Kato, N. Mukaida, K. Matsushima, and H. Tomoike. 2001. Interleukin-8 gene repression by clarithromycin is mediated by the activator protein-1 binding sites in human bronchial epithelial cells. Am. J. Respir. Cell. Mol. Biol. 22:51-60. - PubMed
-
- Blackwell, T. S., and J. W. Christman. 1997. The role of nuclear factor-κB in cytokine gene regulation. Am. J. Respir. Cell. Mol. Biol. 17:3-9. - PubMed
-
- Desaki, M., H. Takizawa, T. Ohtoshi, T. Kasama, K. Kobayashi, T. Sunazuka, Omura, K. Yamamoto, and K. Ito. 2000. Erythromycin suppresses nuclear factor-κB and activator protein-1 activation in human bronchial epithelial cells. Biochem. Biophys. Res. Commun. 267:124-128. - PubMed
-
- Devitt, A., O. D. Moffatt, C. Raykundalia, J. D. Capra, D. L. Simmonda, and C. D. Gregory. 1998. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature 392:505-509. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

