Discrepancies between protease inhibitor concentrations and viral load in reservoirs and sanctuary sites in human immunodeficiency virus-infected patients
- PMID: 12499197
- PMCID: PMC149042
- DOI: 10.1128/AAC.47.1.238-243.2003
Discrepancies between protease inhibitor concentrations and viral load in reservoirs and sanctuary sites in human immunodeficiency virus-infected patients
Abstract
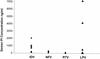
The variable penetration of antiretroviral drugs into sanctuary sites may contribute to the differential evolution of human immunodeficiency virus (HIV) and the emergence of drug resistance. We evaluated the penetration of indinavir, nelfinavir, and lopinavir-ritonavir (lopinavir/r) in the central nervous system, genital tract, and lymphoid tissue and assessed the correlation with residual viral replication. Plasma, cerebrospinal fluid (CSF), semen, and lymph node biopsy samples were collected from 41 HIV-infected patients on stable highly active antiretroviral therapy regimens to determine drug concentrations and HIV RNA levels. When HIV RNA was detectable, sequencing of the reverse transcriptase and protease genes was performed. Ratios of the concentration in semen/concentration in plasma were 1.9 for indinavir, 0.08 for nelfinavir, and 0.07 for lopinavir. Only indinavir was detectable in CSF, with a concentration in CSF/concentration in plasma ratio of 0.17. Differential penetration into lymphoid tissue was observed, with concentration in lymph node tissue/concentration in plasma ratios of 2.07, 0.58, and 0.21 for indinavir, nelfinavir, and lopinavir, respectively. HIV RNA levels were <50 copies/ml in all CSF samples of patients in whom HIV RNA was not detectable in plasma. HIV RNA was detectable in the semen of three patients (two patients receiving nelfinavir and one patient receiving lopinavir/r), and its detection was associated with multiple resistance mutations, while the viral load in plasma was undetectable. HIV RNA was detectable in all lymph node tissue samples. Differential drug penetration was observed among the three protease inhibitors in the sanctuary sites, but there was no correlation between drug levels and HIV RNA levels, suggesting that multiple factors are involved in the persistence of viral reservoirs. Further studies are required to clarify the role and clinical relevance of drug penetration in sanctuaries in terms of long-term efficacy and drug resistance.
Figures


References
-
- Byrn, R. A., and A. A. Kiessling. 1998. Analysis of human immunodeficiency virus in semen: indication of a genetically distinct virus reservoir. J. Reprod. Immunol. 41:161-176. - PubMed
-
- Chun, T. W., L. Carruth, D. Finzi, X. Shen, J. A. DiGiuseppe, H. Taylor, M. Hermankova, K. Chadwick, J. Margolick, T. C. Quinn, Y. H. Kuo, R. Brookmeyer, M. A. Zeiger, P. Barditch-Crovo, and R. F. Siliciano. 1997. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387:183-188. - PubMed
-
- DiStefano, M., J. R. Fiore, L. Monno, A. Lepera, G. Pastore, and G. Angarano. 1999. Detection of multiple drug-resistance-associated pol mutations in cervicovaginal secretions from women. AIDS 13:992-994. - PubMed
-
- Durant, J., P. Clevenbergh, R. Garraffo, P. Halfon, S. Icard, P. Del Giudice, N. Montagne, J. M. Schapiro, and P. Dellamonica. 2000. Importance of protease inhibitor plasma levels in HIV-infected patients treated with genotypic-guided therapy: pharmacologic data from the Viradapt Study. AIDS 14:1333-1339. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

