Worldwide antimicrobial susceptibility patterns and pharmacodynamic comparisons of gatifloxacin and levofloxacin against Streptococcus pneumoniae: report from the Antimicrobial Resistance Rate Epidemiology Study Team
- PMID: 12499204
- PMCID: PMC149036
- DOI: 10.1128/AAC.47.1.292-296.2003
Worldwide antimicrobial susceptibility patterns and pharmacodynamic comparisons of gatifloxacin and levofloxacin against Streptococcus pneumoniae: report from the Antimicrobial Resistance Rate Epidemiology Study Team
Abstract
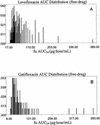
The use of fluoroquinolones for the treatment of community-acquired respiratory tract infection is increasing. Since for Streptococcus pneumoniae a ratio of the 24-h area under the concentration-time curve (AUC(24)) for the agent to the MIC (AUC(24)/MIC) greater than 30 for the fraction of unbound drug (f(u)) is the major pharmacokinetic-pharmacodynamic (PK-PD) parameter correlating with bacterial eradication by fluoroquinolones in nonclinical models of infection and in infected patients, the Antimicrobial Resistance Rate Epidemiology Study Team systematically compared the in vitro susceptibility patterns and estimated the probability of attainment of the PK-PD target ratios for gatifloxacin and levofloxacin against pneumococci worldwide. Monte Carlo simulation was used to estimate the probability that gatifloxacin or levofloxacin would achieve an f(u) AUC(24)/MIC ratio of 30 or greater. A total of 10,978 S. pneumoniae isolates collected from 1997 to 2000, each indexed by site of infection and geographic region (North America, Latin America, Europe, and Asia-Pacific), were used to estimate the probability mass functions of the microbiological activities for each region considered in the analysis. f(u) AUC(24) probability distribution functions were estimated by using data that were part of each product's submission accepted by the Food and Drug Administration. A 10,000-patient simulation was performed for each drug-organism-region combination. The percentages of strains susceptible to each drug by region were as follows: for gatifloxacin, North America, 99.6%; Latin America, 99.8%; Europe, 99.9%; and Asia-Pacific, 99.2%; for levofloxacin, North America, 99.6%; Latin America, 99.8%; Europe, 99.8%; and Asia-Pacific, 99.1%. The MIC at which 50% of isolates are inhibited (MIC(50)) and the MIC(90) of each drug by region were as follows: for gatifloxacin, North America, 0.25 and 0.5 mg/liter, respectively; Latin America, 0.25 and 0.5 mg/liter, respectively; Europe, 0.25 and 0.5 mg/liter, respectively; and Asia-Pacific, 0.25 and 0.5 mg/liter, respectively; for levofloxacin, North America, 1 and 2 mg/liter, respectively; Latin America, 1 and 2 mg/liter, respectively; Europe, 1 and 1 mg/liter, respectively; and Asia-Pacific, 1 and 1 mg/liter, respectively. The probabilities of attaining an f(u) AUC(24)/MIC ratio greater than 30 for each drug by region were as follows: for gatifloxacin, North America, 97.6%; Latin America, 98.3%; Europe, 99.1%; and Asia-Pacific, 98.8%; for levofloxacin, North America, 78.9%; Latin America, 84.1%; Europe, 87.1%; and Asia-Pacific, 86.5%. These results for a very large collection of recent clinical strains demonstrate that, globally, gatifloxacin is two- to fourfold more active than levofloxacin against S. pneumoniae and that gatifloxacin has an overall 14.3% higher probability of achieving clinically important PK-PD target ratios than levofloxacin.
Figures

Similar articles
-
Pharmacodynamic profiling of levofloxacin and gatifloxacin using Monte Carlo simulation for community-acquired isolates of Streptococcus pneumoniae.Am J Med. 2001 Dec 17;111 Suppl 9A:13S-18S; discussion 36S-38S. doi: 10.1016/s0002-9343(01)01026-9. Am J Med. 2001. PMID: 11755438
-
The use of Monte Carlo simulation to examine pharmacodynamic variance of drugs: fluoroquinolone pharmacodynamics against Streptococcus pneumoniae.Diagn Microbiol Infect Dis. 2000 Nov;38(3):151-7. doi: 10.1016/s0732-8893(00)00185-1. Diagn Microbiol Infect Dis. 2000. PMID: 11109013
-
Pharmacodynamic analysis of ceftriaxone, gatifloxacin,and levofloxacin against Streptococcus pneumoniae with the use of Monte Carlo simulation.Pharmacotherapy. 2005 Sep;25(9):1161-7. doi: 10.1592/phco.2005.25.9.1161. Pharmacotherapy. 2005. PMID: 16164390
-
Guide to selection of fluoroquinolones in patients with lower respiratory tract infections.Drugs. 2005;65(7):949-91. doi: 10.2165/00003495-200565070-00004. Drugs. 2005. PMID: 15892589 Free PMC article. Review.
-
Antibiotic pharmacokinetic/pharmacodynamic modelling: MIC, pharmacodynamic indices and beyond.Int J Antimicrob Agents. 2021 Aug;58(2):106368. doi: 10.1016/j.ijantimicag.2021.106368. Epub 2021 May 28. Int J Antimicrob Agents. 2021. PMID: 34058336 Review.
Cited by
-
Evaluating ciprofloxacin dosing for Pseudomonas aeruginosa infection by using clinical outcome-based Monte Carlo simulations.Antimicrob Agents Chemother. 2005 Oct;49(10):4009-14. doi: 10.1128/AAC.49.10.4009-4014.2005. Antimicrob Agents Chemother. 2005. PMID: 16189073 Free PMC article.
-
Update of practice guidelines for the management of community-acquired pneumonia in immunocompetent adults.Clin Infect Dis. 2003 Dec 1;37(11):1405-33. doi: 10.1086/380488. Epub 2003 Nov 3. Clin Infect Dis. 2003. PMID: 14614663 Free PMC article. No abstract available.
-
Antimicrobial treatment guidelines for acute bacterial rhinosinusitis.Otolaryngol Head Neck Surg. 2004 Jan;130(1 Suppl):1-45. doi: 10.1016/j.otohns.2003.12.003. Otolaryngol Head Neck Surg. 2004. PMID: 14726904 Free PMC article. Review.
-
Pharmacokinetics and pharmacodynamics of levofloxacin injection in healthy Chinese volunteers and dosing regimen optimization.J Clin Pharm Ther. 2013 Oct;38(5):394-400. doi: 10.1111/jcpt.12074. Epub 2013 May 24. J Clin Pharm Ther. 2013. PMID: 23701411 Free PMC article. Clinical Trial.
-
Pharmacodynamic profiling of piperacillin in the presence of tazobactam in patients through the use of population pharmacokinetic models and Monte Carlo simulation.Antimicrob Agents Chemother. 2004 Dec;48(12):4718-24. doi: 10.1128/AAC.48.12.4718-4724.2004. Antimicrob Agents Chemother. 2004. PMID: 15561849 Free PMC article. Clinical Trial.
References
-
- Ambrose, P. G., R. C. Owens, and D. M. Grasela. 2000. Antimicrobial pharmacodynamics. Med. Clin. N. Am. 84:1431-1446. - PubMed
-
- Ambrose, P. G., and D. M. Grasela. 2000. The use of Monte Carlo simulation to examine the pharmacodynamic variance of drugs: fluoroquinolone pharmacodynamics against Streptococcus pneumoniae. Diagn. Microbiol. Infect. Dis. 38:151-157. - PubMed
-
- Chen, D. K., A. McGeer, J. C. de Azavedo, and D. E. Low. 1999. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. N. Engl. J. Med. 341:233-239. - PubMed
-
- Craig, W. A. 2001. Pharmacodynamics of antimicrobials: general concepts and application, p. 1-22. In C. H. Nightingale, T. Murakawa, and P. G. Ambrose (ed.), Antimicrobial pharmacodynamics in theory and clinical practice. Marcel-Dekker, Inc., New York, N.Y.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

