Reactivity of reduced [2Fe-2S] ferredoxins parallels host susceptibility to nitroimidazoles
- PMID: 12499206
- PMCID: PMC149022
- DOI: 10.1128/AAC.47.1.302-308.2003
Reactivity of reduced [2Fe-2S] ferredoxins parallels host susceptibility to nitroimidazoles
Abstract
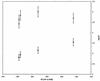
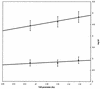
The kinetics of the electron transfer reaction between reduced [2Fe-2S] ferredoxins and select nitroimidazole antimicrobial agents is reported. The ferredoxins from the protozoan Trichomonas vaginalis and the cyanobacterium Anabaena sp. strain 7120 were studied because they are the proximal electron donors to nitroimidazoles in these two organisms with significantly different nitroimidazole susceptibilities. The rates of electron transfer from Anabaena ferredoxin to all nitroimidazoles were 1 to 2 orders of magnitude lower than for T. vaginalis ferredoxin. Quantitative structure-activity analysis of the kinetic data showed that the size of the alkyl substituent on the N-1 position of the imidazole ring strongly influenced the magnitude of the electron transfer rate constant. This implies that the distance between the iron-sulfur cluster and the nitro group of the imidazole is the critical variable in determining the rate of electron transfer. A correlation between the magnitude of the one-electron transfer rate constant with the susceptibility of the host organism to the cytotoxic effects of nitroimidazoles was also discovered. These results demonstrate that reductive activation is the most crucial step in determining the toxicity of nitroimidazoles.
Figures


Similar articles
-
Electrostatic effects in electron transfer reactions of [2Fe-2S] ferredoxins with inorganic reagents.Protein Sci. 1996 Sep;5(9):1793-9. doi: 10.1002/pro.5560050905. Protein Sci. 1996. PMID: 8880903 Free PMC article.
-
Reduction of nitroimidazole derivatives by hydrogenosomal extracts of Trichomonas vaginalis.Mol Biochem Parasitol. 1985 Jan;14(1):29-40. doi: 10.1016/0166-6851(85)90103-3. Mol Biochem Parasitol. 1985. PMID: 3872412
-
Ferredoxin-dependent reduction of nitroimidazole derivatives in drug-resistant and susceptible strains of Trichomonas vaginalis.Biochem Pharmacol. 1986 May 15;35(10):1703-8. doi: 10.1016/0006-2952(86)90327-8. Biochem Pharmacol. 1986. PMID: 3486660
-
Structure-function studies of [2Fe-2S] ferredoxins.J Bioenerg Biomembr. 1994 Feb;26(1):67-88. doi: 10.1007/BF00763220. J Bioenerg Biomembr. 1994. PMID: 8027024 Review.
-
A systematic review of the literature on mechanisms of 5-nitroimidazole resistance in Trichomonas vaginalis.Parasitology. 2020 Nov;147(13):1383-1391. doi: 10.1017/S0031182020001237. Epub 2020 Jul 30. Parasitology. 2020. PMID: 32729451 Free PMC article.
Cited by
-
Alternative pathway of metronidazole activation in Trichomonas vaginalis hydrogenosomes.Antimicrob Agents Chemother. 2005 Dec;49(12):5033-6. doi: 10.1128/AAC.49.12.5033-5036.2005. Antimicrob Agents Chemother. 2005. PMID: 16304169 Free PMC article.
-
Reactions of Plasmodium falciparum Type II NADH: Ubiquinone Oxidoreductase with Nonphysiological Quinoidal and Nitroaromatic Oxidants.Int J Mol Sci. 2025 Mar 11;26(6):2509. doi: 10.3390/ijms26062509. Int J Mol Sci. 2025. PMID: 40141152 Free PMC article.
-
Treatment of infections caused by metronidazole-resistant Trichomonas vaginalis.Clin Microbiol Rev. 2004 Oct;17(4):783-93, table of contents. doi: 10.1128/CMR.17.4.783-793.2004. Clin Microbiol Rev. 2004. PMID: 15489348 Free PMC article. Review.
-
Ferredoxin Gene Mutation in Iranian Trichomonas vaginalis Isolates.Iran J Parasitol. 2013 Jul;8(3):402-7. Iran J Parasitol. 2013. PMID: 24454433 Free PMC article.
-
Nitroimidazole action in Entamoeba histolytica: a central role for thioredoxin reductase.PLoS Biol. 2007 Aug;5(8):e211. doi: 10.1371/journal.pbio.0050211. PLoS Biol. 2007. PMID: 17676992 Free PMC article.
References
-
- Bohme, H., and B. Schrautemeier. 1987. Comparative characterization of ferredoxins from heterocysts and vegetative cells of Anabaena variabilis. Biochim. Biophys. Acta 891:1-7.
-
- Butler, K., H. L. Howes, J. E. Lynch, and D. K. Pirie. 1967. Nitroimidazole derivatives. Relationship between structure and antitrichomonal activity. J. Med. Chem. 10:891-897. - PubMed
-
- Crossnoe, C. R., J. P. Germanas, P. LeMagueres, G. Mustata, and K. L. Krause. 2002. The crystal structure of Trichomonas vaginalis ferredoxin provides insight into metronidazole activation. J. Mol. Biol. 308:503-518. - PubMed
-
- Edwards, D. I., J. H. Tochler, L. D. Dale, D. A. Widdick, D. A., and N. S. Virk. 1990. Effects on DNA of bioreducible nitroimidazole and benzotriazine drugs, p. 275-283. In G. E. Adams, A. Breccia, M. Fielden, and P. Wardman, (ed.), Selective activation of drugs by redox processes. Plenum Press, New York, N.Y.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

