Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin
- PMID: 12499208
- PMCID: PMC148957
- DOI: 10.1128/AAC.47.1.317-323.2003
Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin
Abstract
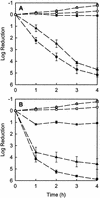
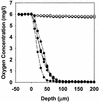
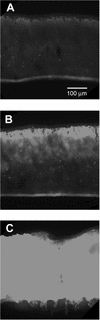
The roles of slow antibiotic penetration, oxygen limitation, and low metabolic activity in the tolerance of Pseudomonas aeruginosa in biofilms to killing by antibiotics were investigated in vitro. Tobramycin and ciprofloxacin penetrated biofilms but failed to effectively kill the bacteria. Bacteria in colony biofilms survived prolonged exposure to either 10 micro g of tobramycin ml(-1)or 1.0 micro g of ciprofloxacin ml(-1). After 100 h of antibiotic treatment, during which the colony biofilms were transferred to fresh antibiotic-containing plates every 24 h, the log reduction in viable cell numbers was only 0.49 +/- 0.18 for tobramycin and 1.42 +/- 0.03 for ciprofloxacin. Antibiotic permeation through colony biofilms, indicated by a diffusion cell bioassay, demonstrated that there was no acceleration in bacterial killing once the antibiotics penetrated the biofilms. These results suggested that limited antibiotic diffusion is not the primary protective mechanism for these biofilms. Transmission electron microscopic observations of antibiotic-affected cells showed lysed, vacuolated, and elongated cells exclusively near the air interface in antibiotic-treated biofilms, suggesting a role for oxygen limitation in protecting biofilm bacteria from antibiotics. To test this hypothesis, a microelectrode analysis was performed. The results demonstrated that oxygen penetrated 50 to 90 micro m into the biofilm from the air interface. This oxic zone correlated to the region of the biofilm where an inducible green fluorescent protein was expressed, indicating that this was the active zone of bacterial metabolic activity. These results show that oxygen limitation and low metabolic activity in the interior of the biofilm, not poor antibiotic penetration, are correlated with antibiotic tolerance of this P. aeruginosa biofilm system.
Figures



References
-
- Brown, M. R. W., D. G. Allison, and P. Gilbert. 1988. Resistance of bacterial biofilms to antibiotics: a growth-rate related effect? J. Antimicrob. Chemother. 22:777-783. - PubMed
-
- Cormack, B. P., R. H. Valdivia, and S. Falkow. 1996. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33-38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

