The prepower stroke conformation of myosin V
- PMID: 12499355
- PMCID: PMC2173995
- DOI: 10.1083/jcb.200208172
The prepower stroke conformation of myosin V
Abstract
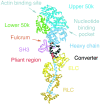
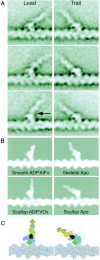
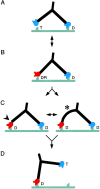
We have used electron microscopy and single-particle image processing to study head conformation in myosin V molecules. We find that in the presence of ATP, many heads have a sharply angled conformation that is rare in its absence. The sharply angled conformation is similar to a myosin II atomic structure proposed to mimic the prepower stroke state. The leading head in molecules attached to actin by both heads has a similar conformation, but is also sharply angled in a second plane by tethering through the trail head. The lead head lever joins the motor domain approximately 5 nm axially from where it joins the trail motor. These positions locate the converter subdomain and show the lead motor is in the prepower stroke conformation. Tethering by the trail head places the lead head motor domain at the correct axial position along the actin for binding, but at the wrong orientation. Attachment is achieved either by bending the lead head lever throughout its length or at the pliant point. The microscopy shows that most of the walking stride is produced by changes in lever angle brought about by converter movement, but is augmented by distortion produced by thermal energy.
Figures




Similar articles
-
Direct observation of the myosin Va recovery stroke that contributes to unidirectional stepping along actin.PLoS Biol. 2011 Apr;9(4):e1001031. doi: 10.1371/journal.pbio.1001031. Epub 2011 Apr 12. PLoS Biol. 2011. PMID: 21532738 Free PMC article.
-
Reverse conformational changes of the light chain-binding domain of myosin V and VI processive motor heads during and after hydrolysis of ATP by small-angle X-ray solution scattering.J Mol Biol. 2009 Sep 18;392(2):420-35. doi: 10.1016/j.jmb.2009.07.013. Epub 2009 Jul 14. J Mol Biol. 2009. PMID: 19607837 Free PMC article.
-
A structural state of the myosin V motor without bound nucleotide.Nature. 2003 Sep 25;425(6956):419-23. doi: 10.1038/nature01927. Nature. 2003. PMID: 14508494
-
Visualizing myosin's power stroke in muscle contraction.J Cell Sci. 2000 Oct;113 ( Pt 20):3551-62. doi: 10.1242/jcs.113.20.3551. J Cell Sci. 2000. PMID: 11017871 Review.
-
Myosin motors: missing structures and hidden springs.Curr Opin Struct Biol. 2001 Apr;11(2):182-94. doi: 10.1016/s0959-440x(00)00188-3. Curr Opin Struct Biol. 2001. PMID: 11297926 Review.
Cited by
-
Unconstrained steps of myosin VI appear longest among known molecular motors.Biophys J. 2004 Jun;86(6):3804-10. doi: 10.1529/biophysj.103.037416. Biophys J. 2004. PMID: 15189876 Free PMC article.
-
Similarities and differences between frozen-hydrated, rigor acto-S1 complexes of insect flight and chicken skeletal muscles.J Mol Biol. 2008 Sep 5;381(3):519-28. doi: 10.1016/j.jmb.2008.06.029. Epub 2008 Jun 17. J Mol Biol. 2008. PMID: 18588896 Free PMC article.
-
Nucleotide-dependent shape changes in the reverse direction motor, myosin VI.Biophys J. 2010 Nov 17;99(10):3336-44. doi: 10.1016/j.bpj.2010.09.014. Biophys J. 2010. PMID: 21081082 Free PMC article.
-
Myosin regulatory domain orientation in skeletal muscle fibers: application of novel electron paramagnetic resonance spectral decomposition and molecular modeling methods.Biophys J. 2004 May;86(5):3030-41. doi: 10.1016/S0006-3495(04)74352-0. Biophys J. 2004. PMID: 15111417 Free PMC article.
-
Swinging lever mechanism of myosin directly shown by time-resolved cryo-EM.Nature. 2025 Jun;642(8067):519-526. doi: 10.1038/s41586-025-08876-5. Epub 2025 Apr 9. Nature. 2025. PMID: 40205053 Free PMC article.
References
-
- Bauer, C.B., H.M. Holden, J.B. Thoden, R. Smith, and I. Rayment. 2000. X-ray structures of the apo and MgATP-bound states of Dictyostelium discoideum myosin motor domain. J. Biol. Chem. 275:38494–38499. - PubMed
-
- de la Cruz, E.M., E.M. Ostap, and H.L. Sweeney. 2001. Kinetic mechanism and regulation of myosin VI. J. Biol. Chem. 276:32373–32381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

