Migration of nerve growth cones requires detergent-resistant membranes in a spatially defined and substrate-dependent manner
- PMID: 12499360
- PMCID: PMC2173975
- DOI: 10.1083/jcb.200209077
Migration of nerve growth cones requires detergent-resistant membranes in a spatially defined and substrate-dependent manner
Abstract
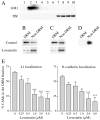
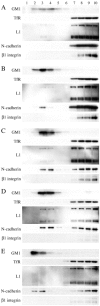
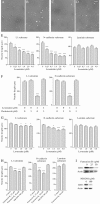
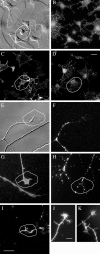
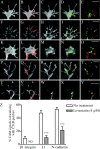
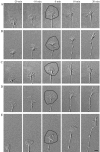
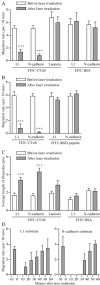
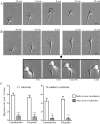
Motility of nerve growth cones (GCs) is regulated by region-specific activities of cell adhesion molecules (CAMs). CAM activities could be modified by their localization to detergent-resistant membranes (DRMs), specialized microdomains enriched in signaling molecules. This paper deals with a question of whether DRMs are involved in GC migration stimulated by three CAMs; L1, N-cadherin (Ncad), and beta1 integrin. We demonstrate that L1 and Ncad are present in DRMs, whereas beta1 integrin is exclusively detected in non-DRMs of neurons and that localization of L1 and Ncad to DRMs is developmentally regulated. GC migration mediated by L1 and Ncad but not by beta1 integrin is inhibited after DRM disruption by micro-scale chromophore-assisted laser inactivation (micro-CALI) of GM1 gangliosides or by pharmacological treatments that deplete cellular cholesterol or sphingolipids, essential components for DRMs. Characteristic morphology of GCs induced by L1 and Ncad is also affected by micro-CALI-mediated DRM disruption. Micro-CALI within the peripheral domain of GCs, or even within smaller areas such as the filopodia and the lamellipodia, is sufficient to impair their migration. However, micro-CALI within the central domain does not affect GC migration. These results demonstrate the region-specific involvement of DRMs in CAM-dependent GC behavior.
Figures

Similar articles
-
Isolation and characterization of detergent-resistant microdomains responsive to NCAM-mediated signaling from growth cones.Mol Cell Neurosci. 2002 Jan;19(1):18-31. doi: 10.1006/mcne.2001.1060. Mol Cell Neurosci. 2002. PMID: 11817895
-
Structure of the ceramide moiety of GM1 ganglioside determines its occurrence in different detergent-resistant membrane domains in HL-60 cells.Biochemistry. 2003 Jun 3;42(21):6608-19. doi: 10.1021/bi0206309. Biochemistry. 2003. PMID: 12767245
-
Drosophila N-cadherin functions in the first stage of the two-stage layer-selection process of R7 photoreceptor afferents.Development. 2005 Mar;132(5):953-63. doi: 10.1242/dev.01661. Epub 2005 Jan 26. Development. 2005. PMID: 15673571
-
Micro-scale chromophore-assisted laser inactivation of nerve growth cone proteins.Microsc Res Tech. 2000 Jan 15;48(2):97-106. doi: 10.1002/(SICI)1097-0029(20000115)48:2<97::AID-JEMT5>3.0.CO;2-G. Microsc Res Tech. 2000. PMID: 10649510 Review.
-
Chromophore-assisted laser inactivation--towards a spatiotemporal-functional analysis of proteins, and the ablation of chromatin, organelle and cell function.J Cell Sci. 2014 Apr 15;127(Pt 8):1621-9. doi: 10.1242/jcs.144527. J Cell Sci. 2014. PMID: 24737873 Review.
Cited by
-
Choline supplementation prevents the effects of bilirubin on cerebellar-mediated behavior in choline-restricted Gunn rat pups.Pediatr Res. 2021 May;89(6):1414-1419. doi: 10.1038/s41390-020-01187-7. Epub 2020 Oct 7. Pediatr Res. 2021. PMID: 33027804 Free PMC article.
-
Desmosome assembly and disassembly are membrane raft-dependent.PLoS One. 2014 Jan 30;9(1):e87809. doi: 10.1371/journal.pone.0087809. eCollection 2014. PLoS One. 2014. PMID: 24498201 Free PMC article.
-
Nuclear factor I coordinates multiple phases of cerebellar granule cell development via regulation of cell adhesion molecules.J Neurosci. 2007 Jun 6;27(23):6115-27. doi: 10.1523/JNEUROSCI.0180-07.2007. J Neurosci. 2007. PMID: 17553984 Free PMC article.
-
L1 cell adhesion molecule signaling is inhibited by ethanol in vivo.Alcohol Clin Exp Res. 2013 Mar;37(3):383-9. doi: 10.1111/j.1530-0277.2012.01944.x. Epub 2012 Oct 10. Alcohol Clin Exp Res. 2013. PMID: 23050935 Free PMC article.
-
NrCAM coupling to the cytoskeleton depends on multiple protein domains and partitioning into lipid rafts.Mol Biol Cell. 2004 Oct;15(10):4695-709. doi: 10.1091/mbc.e04-03-0171. Epub 2004 Jul 14. Mol Biol Cell. 2004. PMID: 15254265 Free PMC article.
References
-
- Anderson, R.G., and K. Jacobson. 2002. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 296:1821–1825. - PubMed
-
- Aruga, J., N. Yokota, M. Hashimoto, T. Furuichi, M. Fukuda, and K. Mikoshiba. 1994. A novel zinc finger protein, zic, is involved in neurogenesis, especially in the cell lineage of cerebellar granule cells. J. Neurochem. 63:1880–1890. - PubMed
-
- Bickel, P.E., P.E. Scherer, J.E. Schnitzer, P. Oh, M.P. Lisanti, and H.F. Lodish. 1997. Flotillin and epidermal surface antigen define a new family of caveolae-associated integral membrane proteins. J. Biol. Chem. 272:13793–13802. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

