Neurite branching on deformable substrates
- PMID: 12499839
- PMCID: PMC2408859
- DOI: 10.1097/00001756-200212200-00007
Neurite branching on deformable substrates
Abstract
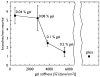
The mechanical properties of substrates underlying cells can have profound effects on cell structure and function. To examine the effect of substrate deformability on neuronal cell growth, protein-laminated polyacrylamide gels were prepared with differing amounts of bisacrylamide to generate substrates of varying deformability with elastic moduli ranging from 500 to 5500 dyne/cm. Mouse spinal cord primary neuronal cells were plated on the gels and allowed to grow and extend neurites for several weeks in culture. While neurons grew well on the gels, glia, which are normally co-cultured with the neurons, did not survive on these deformable substrates even though the chemical environment was permissive for their growth. Substrate flexibility also had a significant effect on neurite branching. Neurons grown on softer substrates formed more than three times as many branches as those grown on stiffer gels. These results show that mechanical properties of the substrate specifically direct the formation of neurite branches, which are critical for appropriate synaptic connections during development and regeneration.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

