The role of neutralizing antibodies for mouse mammary tumor virus transmission and mammary cancer development
- PMID: 12502785
- PMCID: PMC140926
- DOI: 10.1073/pnas.0134988100
The role of neutralizing antibodies for mouse mammary tumor virus transmission and mammary cancer development
Abstract
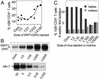
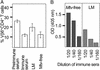
Mouse mammary tumor virus (MMTV) infection establishes chronic germinal centers and a lifelong neutralizing Ab response. We show that removal of the draining lymph node after establishment of the germinal center reaction led to complete loss of neutralizing Abs despite comparable infection levels in peripheral lymphocytes. Importantly, in the absence of neutralization, only the exocrine organs mammary gland, salivary gland, pancreas, and skin showed strikingly increased infection, resulting in accelerated mammary tumor development. Induction of stronger neutralization did not influence chronic infection levels of peripheral lymphoid organs but strongly inhibited mammary gland infection and virus transmission to the next generation. Taken together, we provide evidence that a tight equilibrium in virus neutralization allows limited infection of exocrine organs and controls cancer development in susceptible mouse strains. These experiments show that a strong neutralizing Ab response induced after infection is not able to control lymphoid MMTV infection. Strong neutralization, however, is capable of blocking amplification of mammary gland infection, tumor development, and virus transmission to the next generation. The results also indicate a role of neutralization in natural resistance to MMTV infection.
Figures
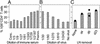


Similar articles
-
Passive immunization with neutralizing antibodies interrupts the mouse mammary tumor virus life cycle.J Virol. 2003 Sep;77(17):9369-77. doi: 10.1128/jvi.77.17.9369-9377.2003. J Virol. 2003. PMID: 12915552 Free PMC article.
-
C3H/HeN mammary tumor-bearing mice develop type-specific neutralizing antibodies and group-specific precipitating antibodies for the mouse mammary tumor virus.J Virol. 1980 Jan;33(1):123-8. doi: 10.1128/JVI.33.1.123-128.1980. J Virol. 1980. PMID: 6154146 Free PMC article.
-
Mice with spontaneous mammary tumors develop type-specific neutralizing and cytotoxic antibodies against the mouse mammary tumor virus envelope protein gp52.J Virol. 1979 Oct;32(1):131-9. doi: 10.1128/JVI.32.1.131-139.1979. J Virol. 1979. PMID: 94356 Free PMC article.
-
Immune response to MMTV infection.Front Biosci. 2007 Jan 1;12:1594-609. doi: 10.2741/2172. Front Biosci. 2007. PMID: 17127406 Review.
-
Humoral and cellular immune responses to mouse mammary tumor virus (MMTV) in experimental animal models (review).Anticancer Res. 1984 Jul-Oct;4(4-5):305-12. Anticancer Res. 1984. PMID: 6091529 Review.
Cited by
-
Endogenous MMTV proviruses induce susceptibility to both viral and bacterial pathogens.PLoS Pathog. 2006 Dec;2(12):e128. doi: 10.1371/journal.ppat.0020128. PLoS Pathog. 2006. PMID: 17140288 Free PMC article.
-
Genetic Control of Neonatal Immune Tolerance to an Exogenous Retrovirus.J Virol. 2020 Nov 23;94(24):e01608-20. doi: 10.1128/JVI.01608-20. Print 2020 Nov 23. J Virol. 2020. PMID: 32999021 Free PMC article.
-
Passive immunization with neutralizing antibodies interrupts the mouse mammary tumor virus life cycle.J Virol. 2003 Sep;77(17):9369-77. doi: 10.1128/jvi.77.17.9369-9377.2003. J Virol. 2003. PMID: 12915552 Free PMC article.
-
MHC Class II Presentation Is Affected by Polymorphism in the H2-Ob Gene and Additional Loci.J Immunol. 2021 Jul 1;207(1):5-14. doi: 10.4049/jimmunol.2100061. Epub 2021 Jun 16. J Immunol. 2021. PMID: 34135064 Free PMC article.
-
Neutralizing Antibody Responses to Viral Infections Are Linked to the Non-classical MHC Class II Gene H2-Ob.Immunity. 2017 Aug 15;47(2):310-322.e7. doi: 10.1016/j.immuni.2017.07.013. Immunity. 2017. PMID: 28813660 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

