Proline 78 is crucial for human immunodeficiency virus type 1 Nef to down-regulate class I human leukocyte antigen
- PMID: 12502873
- PMCID: PMC140770
- DOI: 10.1128/jvi.77.2.1589-1594.2003
Proline 78 is crucial for human immunodeficiency virus type 1 Nef to down-regulate class I human leukocyte antigen
Abstract
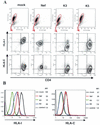
Human immunodeficiency virus type 1 Nef down-regulates human leukocyte antigen class I (HLA-I) in T lymphocytes, and the down-regulation involves the Nef proline-rich domain (PRD) containing four prolines at positions 69, 72, 75, and 78. We used a Sendai virus vector with nef and examined regulation by Nef of HLA-I and CD4 in suspension cultures of cells such as T lymphocytes. Analyses of a series of PRD substitution mutants indicated that, because the substitution of Pro78 with Ala abolished down-regulation of HLA-I but not of CD4, Pro78 is important for HLA-I down-regulation in T lymphocytes.
Figures


References
-
- Aiken, C., J. Konner, N. R. Landau, M. E. Lenburg, and D. Trono. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76:853-864. - PubMed
-
- Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. - PubMed
-
- Collins, K. L., and D. Baltimore. 1999. HIV's evasion of the cellular immune response. Immunol. Rev. 168:65-74. - PubMed
-
- Collins, K. L., B. K. Chen, S. A. Kalams, B. D. Walker, and D. Baltimore. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391:397-401. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

