Expression of Th1-mediated immunity in mouse lungs induces a Mycobacterium tuberculosis transcription pattern characteristic of nonreplicating persistence
- PMID: 12506197
- PMCID: PMC140939
- DOI: 10.1073/pnas.0136863100
Expression of Th1-mediated immunity in mouse lungs induces a Mycobacterium tuberculosis transcription pattern characteristic of nonreplicating persistence
Abstract
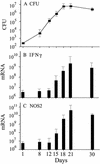
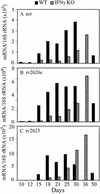
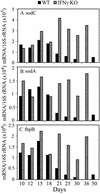
The lung is the primary target of infection with Mycobacterium tuberculosis. It is well established that, in mouse lung, expression of adaptive, Th1-mediated host immunity inhibits further multiplication of M. tuberculosis. Here, real-time RT-PCR was used to define the pattern of expression against time of lung infection of key genes involved in Th1-mediated immunity and of selected genes of M. tuberculosis. Inhibition of bacterial multiplication was preceded by increased mRNA synthesis for IFN-gamma and inducible NO synthase (NOS2) and by NOS2 protein synthesis in infected macrophages. Concurrently, the pattern of transcription of bacterial genes underwent dramatic changes. mRNA synthesis increased for alpha-crystallin (acr), rv2626c, and rv2623 and decreased for superoxide dismutase C (sodC), sodA, and fibronectin-binding protein B (fbpB). This pattern of M. tuberculosis transcription is characteristic of the nonreplicating persistence [Wayne, L. G. & Sohaskey, C. D. (2001) Annu. Rev. Microbiol. 55, 139-163] associated with adaptation of tubercle bacilli to hypoxia in vitro. Based on this similarity, we infer that host immunity induces bacterial growth arrest. In IFN-gamma gene-deleted mice, bacterial growth was not controlled; NOS2 protein was not detected in macrophages; sodC, sodA, and fbpB transcription showed no decrease; and acr, rv2626c, and rv2623 transcription increased only at the terminal stages of lung pathology. These findings define the transcription signature of M. tuberculosis as it transitions from growth to persistence in the mouse lung. The bacterial transcription changes measured at onset of Th1-mediated immunity are likely induced, directly or indirectly, by nitric oxide generated by infected macrophages.
Figures

Similar articles
-
Evidence inconsistent with a negative influence of T helper 2 cells on protection afforded by a dominant T helper 1 response against Mycobacterium tuberculosis lung infection in mice.Infect Immun. 2002 Nov;70(11):6436-43. doi: 10.1128/IAI.70.11.6436-6443.2002. Infect Immun. 2002. PMID: 12379724 Free PMC article.
-
Expression levels of Mycobacterium tuberculosis antigen-encoding genes versus production levels of antigen-specific T cells during stationary level lung infection in mice.Immunology. 2006 Jun;118(2):195-201. doi: 10.1111/j.1365-2567.2006.02355.x. Immunology. 2006. PMID: 16771854 Free PMC article.
-
Defects in cell-mediated immunity affect chronic, but not innate, resistance of mice to Mycobacterium avium infection.J Immunol. 1997 May 15;158(10):4822-31. J Immunol. 1997. PMID: 9144497
-
[Up-to-date understanding of tuberculosis immunity].Kekkaku. 2003 Jan;78(1):51-5. Kekkaku. 2003. PMID: 12683337 Review. Japanese.
-
Mycobacterium tuberculosis gene expression during environmental conditions associated with latency.Tuberculosis (Edinb). 2004;84(3-4):138-43. doi: 10.1016/j.tube.2003.12.008. Tuberculosis (Edinb). 2004. PMID: 15207483 Review. No abstract available.
Cited by
-
The intracellular environment of human macrophages that produce nitric oxide promotes growth of mycobacteria.Infect Immun. 2013 Sep;81(9):3198-209. doi: 10.1128/IAI.00611-13. Epub 2013 Jun 17. Infect Immun. 2013. PMID: 23774601 Free PMC article.
-
Transcription of genes involved in sulfolipid and polyacyltrehalose biosynthesis of Mycobacterium tuberculosis in experimental latent tuberculosis infection.PLoS One. 2013;8(3):e58378. doi: 10.1371/journal.pone.0058378. Epub 2013 Mar 5. PLoS One. 2013. PMID: 23472191 Free PMC article.
-
Dormancy phenotype displayed by extracellular Mycobacterium tuberculosis within artificial granulomas in mice.J Exp Med. 2004 Sep 6;200(5):647-57. doi: 10.1084/jem.20040646. J Exp Med. 2004. PMID: 15353557 Free PMC article.
-
Immunogenicity of eight dormancy regulon-encoded proteins of Mycobacterium tuberculosis in DNA-vaccinated and tuberculosis-infected mice.Infect Immun. 2007 Feb;75(2):941-9. doi: 10.1128/IAI.01137-06. Epub 2006 Dec 4. Infect Immun. 2007. PMID: 17145953 Free PMC article.
-
Deletion of the Mycobacterium tuberculosis resuscitation-promoting factor Rv1009 gene results in delayed reactivation from chronic tuberculosis.Infect Immun. 2006 May;74(5):2985-95. doi: 10.1128/IAI.74.5.2985-2995.2006. Infect Immun. 2006. PMID: 16622237 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

