NDP kinase 2 interacts with two oxidative stress-activated MAPKs to regulate cellular redox state and enhances multiple stress tolerance in transgenic plants
- PMID: 12506203
- PMCID: PMC140977
- DOI: 10.1073/pnas.252641899
NDP kinase 2 interacts with two oxidative stress-activated MAPKs to regulate cellular redox state and enhances multiple stress tolerance in transgenic plants
Abstract
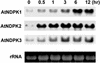
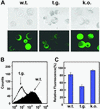
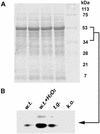
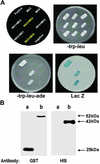
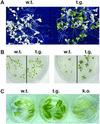
NDP kinases (NDPKs) are multifunctional proteins that regulate a variety of eukaryotic cellular activities, including cell proliferation, development, and differentiation. However, much less is known about the functional significance of NDPKs in plants. We show here that NDPK is associated with H(2)O(2)-mediated mitogen-activated protein kinase signaling in plants. H(2)O(2) stress strongly induces the expression of the NDPK2 gene in Arabidopsis thaliana (AtNDPK2). Proteins from transgenic plants overexpressing AtNDPK2 showed high levels of autophosphorylation and NDPK activity, and they have lower levels of reactive oxygen species (ROS) than wild-type plants. Mutants lacking AtNDPK2 had higher levels of ROS than wild type. H(2)O(2) treatment induced the phosphorylation of two endogenous proteins whose molecular weights suggested they are AtMPK3 and AtMPK6, two H(2)O(2)-activated A. thaliana mitogen-activated protein kinases. In the absence of H(2)O(2) treatment, phosphorylation of these proteins was slightly elevated in plants overexpressing AtNDPK2 but markedly decreased in the AtNDPK2 deletion mutant. Yeast two-hybrid and in vitro protein pull-down assays revealed that AtNDPK2 specifically interacts with AtMPK3 and AtMPK6. Furthermore, AtNDPK2 also enhances the myelin basic protein phosphorylation activity of AtMPK3 in vitro. Finally, constitutive overexpression of AtNDPK2 in Arabidopsis plants conferred an enhanced tolerance to multiple environmental stresses that elicit ROS accumulation in situ. Thus, AtNDPK2 appears to play a previously uncharacterized regulatory role in H(2)O(2)-mediated MAPK signaling in plants.
Figures


References
-
- Finkel T, Holbrook N J. Nature. 2000;408:239–247. - PubMed
-
- Kannan K, Jain S K. Pathophysiology. 2000;7:153–163. - PubMed
-
- Hallwell B, Gutteridge J M C. Free Radicals in Biology and Medicine. 3rd Ed. New York: Oxford Univ. Press; 2002.
-
- Lamb C, Dixon R A. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:251–275. - PubMed
-
- Bolwell G P, Wojtaszek P. Physiol Mol Plant Pathol. 1997;51:347–366.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

