Catalysis of electron transfer during activation of O2 by the flavoprotein glucose oxidase
- PMID: 12506204
- PMCID: PMC404145
- DOI: 10.1073/pnas.252644599
Catalysis of electron transfer during activation of O2 by the flavoprotein glucose oxidase
Abstract
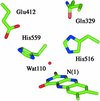
Two prototropic forms of glucose oxidase undergo aerobic oxidation reactions that convert FADH(-) to FAD and form H(2)O(2) as a product. Limiting rate constants of k(cat)K(M)(O(2)) = (5.7 +/- 1.8) x 10(2) M(-1).s(-1) and k(cat)K(M)(O(2)) = (1.5 +/- 0.3) x 10(6) M(-1).s(-1) are observed at high and low pH, respectively. Reactions exhibit oxygen-18 kinetic isotope effects but no solvent kinetic isotope effects, consistent with mechanisms of rate-limiting electron transfer from flavin to O(2). Site-directed mutagenesis studies reveal that the pH dependence of the rates is caused by protonation of a highly conserved histidine in the active site. Temperature studies (283-323 K) indicate that protonation of His-516 results in a reduction of the activation energy barrier by 6.0 kcal.mol(-1) (0.26 eV). Within the context of Marcus theory, catalysis of electron transfer is attributed to a 19-kcal.mol(-1) (0.82 eV) decrease in the reorganization energy and a much smaller 2.2-kcal.mol(-1) (0.095 eV) enhancement of the reaction driving force. An explanation is advanced that is based on changes in outer-sphere reorganization as a function of pH. The active site is optimized at low pH, but not at high pH or in the H516A mutant where rates resemble the uncatalyzed reaction in solution.
Figures

Similar articles
-
Oxygen isotope effects on electron transfer to O2 probed using chemically modified flavins bound to glucose oxidase.J Am Chem Soc. 2004 Nov 24;126(46):15120-31. doi: 10.1021/ja047050e. J Am Chem Soc. 2004. PMID: 15548009
-
Nature of oxygen activation in glucose oxidase from Aspergillus niger: the importance of electrostatic stabilization in superoxide formation.Biochemistry. 1999 Jun 29;38(26):8572-81. doi: 10.1021/bi990044o. Biochemistry. 1999. PMID: 10387105
-
Evidence for concerted electron proton transfer in charge recombination between FADH- and 306Trp• in Escherichia coli photolyase.J Am Chem Soc. 2011 May 25;133(20):7824-36. doi: 10.1021/ja2001488. Epub 2011 May 2. J Am Chem Soc. 2011. PMID: 21534528
-
On the catalytic role of the conserved active site residue His466 of choline oxidase.Biochemistry. 2005 Jan 25;44(3):893-904. doi: 10.1021/bi048056j. Biochemistry. 2005. PMID: 15654745
-
Glucose oxidase from Aspergillus niger: the mechanism of action with molecular oxygen, quinones, and one-electron acceptors.Int J Biochem Cell Biol. 2005 Apr;37(4):731-50. doi: 10.1016/j.biocel.2004.10.014. Int J Biochem Cell Biol. 2005. PMID: 15694834 Review.
Cited by
-
Oxygen reactivity of PutA from Helicobacter species and proline-linked oxidative stress.J Bacteriol. 2006 Feb;188(4):1227-35. doi: 10.1128/JB.188.4.1227-1235.2006. J Bacteriol. 2006. PMID: 16452403 Free PMC article.
-
Contribution of flavin covalent linkage with histidine 99 to the reaction catalyzed by choline oxidase.J Biol Chem. 2009 Jun 19;284(25):16990-16997. doi: 10.1074/jbc.M109.003715. Epub 2009 Apr 27. J Biol Chem. 2009. PMID: 19398559 Free PMC article.
-
Molecular Basis for Converting (2S)-Methylsuccinyl-CoA Dehydrogenase into an Oxidase.Molecules. 2017 Dec 28;23(1):68. doi: 10.3390/molecules23010068. Molecules. 2017. PMID: 29283425 Free PMC article.
-
Investigating inner-sphere reorganization via secondary kinetic isotope effects in the C-H cleavage reaction catalyzed by soybean lipoxygenase: tunneling in the substrate backbone as well as the transferred hydrogen.J Am Chem Soc. 2011 Jan 26;133(3):430-9. doi: 10.1021/ja1050742. Epub 2010 Dec 30. J Am Chem Soc. 2011. PMID: 21192631 Free PMC article.
-
Peroxo and Superoxo Moieties Bound to Copper Ion: Electron-Transfer Equilibrium with a Small Reorganization Energy.J Am Chem Soc. 2016 Jun 8;138(22):7055-66. doi: 10.1021/jacs.6b02404. Epub 2016 May 26. J Am Chem Soc. 2016. PMID: 27228314 Free PMC article.
References
-
- Massey V. & Hemmerich, P. (1980) Biochem. Soc. Trans. 8, 246-257. - PubMed
-
- Hille R. & Anderson, R. F. (2001) J. Biol. Chem. 276, 31193-31201. - PubMed
-
- Ghisla S. & Massey, V. (1989) Eur. J. Biochem. 181, 1-17. - PubMed
-
- Fitzpatrick P. F. (2001) Acc. Chem. Res. 34, 299-307. - PubMed
-
- Hemmerich P., Nagelshneider, G. & Veeger, C. (1970) FEBS Lett. 8, 69-83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

