Up-regulation of macrophage migration inhibitory factor gene and protein expression in glial tumor cells during hypoxic and hypoglycemic stress indicates a critical role for angiogenesis in glioblastoma multiforme
- PMID: 12507885
- PMCID: PMC1851138
- DOI: 10.1016/S0002-9440(10)63793-5
Up-regulation of macrophage migration inhibitory factor gene and protein expression in glial tumor cells during hypoxic and hypoglycemic stress indicates a critical role for angiogenesis in glioblastoma multiforme
Abstract
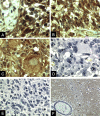
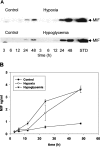
Glioblastoma multiforme (GBM) is the most malignant variant of human glial tumors. A prominent feature of this tumor is the occurrence of necrosis and vascular proliferation. The regulation of glial neovascularization is still poorly understood and the characterization of factors involved in this process is of major clinical interest. Macrophage migration inhibitory factor (MIF) is a pleiotropic cytokine released by leukocytes and by a variety of cells outside of the immune system. Recent work has shown that MIF may function to regulate cellular differentiation and proliferation in normal and tumor-derived cell lines, and may also contribute to the neovascularization of tumors. Our immunohistological analysis of MIF distribution in GBM tissues revealed the strong MIF protein accumulation in close association with necrotic areas and in tumor cells surrounding blood vessels. In addition, MIF expression was frequently associated with the presence of the tumor-suppressor gene p53. To substantiate the concept that MIF might be involved in the regulation of angiogenesis in GBM, we analyzed the MIF gene and protein expression under hypoxic and hypoglycemic stress conditions in vitro. Northern blot analysis showed a clear increase of MIF mRNA after hypoxia and hypoglycemia. We could also demonstrate that the increase of MIF transcripts on hypoxic stress can be explained by a profound transcriptional activation of the MIF gene. In parallel to the increase of MIF transcripts, we observed a significant rise in extracellular MIF protein on angiogenic stimulation. The data of our preliminary study suggest that the up-regulation of MIF expression during hypoxic and hypoglycemic stress might play a critical role for the neovascularization of glial tumors.
Figures



References
-
- Plate KH, Breier G, Weich HA, Risau W: Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature , 359:845-848 - PubMed
-
- David JR, Bozza M, Carini C: Macrophage migration inhibitory factor. Aggraval BB Gutterman JU eds. Human Cytokines. Handbook for Basic and Clinical Research, 1996, vol 2.:pp 222-256 Blackwell Science, Cambridge
-
- Bucala R: MIF rediscovered: cytokine, pituitary hormone, and glucocorticoid regulator of the immune response. EMBO J 1996, 10:1607-1613 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

