Genotype-phenotype relationship in human ATP6i-dependent autosomal recessive osteopetrosis
- PMID: 12507890
- PMCID: PMC1851135
- DOI: 10.1016/S0002-9440(10)63798-4
Genotype-phenotype relationship in human ATP6i-dependent autosomal recessive osteopetrosis
Abstract
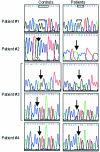
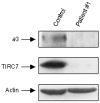
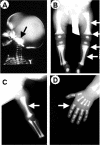
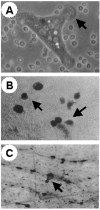
Autosomal-recessive osteopetrosis is a severe genetic disease caused by osteoclast failure. Approximately 50% of the patients harbor mutations of the ATP6i gene, encoding for the osteoclast-specific a3 subunit of V-ATPase. We found inactivating ATP6i mutations in four patients, and three of these were novel. Patients shared macrocephaly, growth retardation and optic nerve alteration, osteosclerotic and endobone patterns, and high alkaline phosphatase and parathyroid hormone levels. Bone biopsies revealed primary spongiosa lined with active osteoblasts and high numbers of tartrate-resistant acid phosphatase (TRAP)-positive, a3 subunit-negative, morphologically unremarkable osteoclasts, some of which located in shallow Howship lacunae. Scarce hematopoietic cells and abundant fibrous tissue containing TRAP-positive putative osteoclast precursors were noted. In vitro osteoclasts were a3-negative, morphologically normal, with prominent clear zones and actin rings, and TRAP activity more elevated than in control patients. Podosomes, alphaVbeta3 receptor, c-Src, and PYK2 were unremarkable. Consistent with the finding in the bone biopsies, these cells excavated pits faintly stained with toluidine blue, indicating inefficient bone resorption. Bone marrow transplantation was successful in all patients, and posttransplant osteoclasts showed rescue of a3 subunit immunoreactivity.
Figures






Similar articles
-
Osteoclast morphology in autosomal recessive malignant osteopetrosis due to a TCIRG1 gene mutation.Pediatr Pathol Mol Med. 2003 Jan-Feb;22(1):3-9. doi: 10.1080/pdp.22.1.3.9. Pediatr Pathol Mol Med. 2003. PMID: 12687885
-
In vitro differentiation of CD14 cells from osteopetrotic subjects: contrasting phenotypes with TCIRG1, CLCN7, and attachment defects.J Bone Miner Res. 2004 Aug;19(8):1329-38. doi: 10.1359/JBMR.040403. Epub 2004 Apr 5. J Bone Miner Res. 2004. PMID: 15231021
-
Novel c.G630A TCIRG1 mutation causes aberrant splicing resulting in an unusually mild form of autosomal recessive osteopetrosis.J Cell Biochem. 2019 Oct;120(10):17180-17193. doi: 10.1002/jcb.28979. Epub 2019 May 20. J Cell Biochem. 2019. PMID: 31111556
-
Human osteopetroses and the osteoclast V-H+-ATPase enzyme system.Front Biosci. 2005 Sep 1;10:2940-54. doi: 10.2741/1750. Front Biosci. 2005. PMID: 15970548 Review.
-
Dysosteosclerosis presents as an "osteoclast-poor" form of osteopetrosis: comprehensive investigation of a 3-year-old girl and literature review.J Bone Miner Res. 2010 Nov;25(11):2527-39. doi: 10.1002/jbmr.131. J Bone Miner Res. 2010. PMID: 20499338 Free PMC article. Review.
Cited by
-
Identification of inhibitors of vacuolar proton-translocating ATPase pumps in yeast by high-throughput screening flow cytometry.Anal Biochem. 2010 Mar 15;398(2):203-11. doi: 10.1016/j.ab.2009.12.020. Epub 2009 Dec 14. Anal Biochem. 2010. PMID: 20018164 Free PMC article.
-
Fetal liver cells transplanted in utero rescue the osteopetrotic phenotype in the oc/oc mouse.Am J Pathol. 2009 Mar;174(3):727-35. doi: 10.2353/ajpath.2009.080688. Epub 2009 Feb 13. Am J Pathol. 2009. PMID: 19218349 Free PMC article.
-
Versatile roles of V-ATPases accessory subunit Ac45 in osteoclast formation and function.PLoS One. 2011;6(11):e27155. doi: 10.1371/journal.pone.0027155. Epub 2011 Nov 4. PLoS One. 2011. PMID: 22087256 Free PMC article.
-
Combinatorial Surface Roughness Effects on Osteoclastogenesis and Osteogenesis.ACS Appl Mater Interfaces. 2018 Oct 31;10(43):36652-36663. doi: 10.1021/acsami.8b10992. Epub 2018 Oct 16. ACS Appl Mater Interfaces. 2018. PMID: 30270615 Free PMC article.
-
Involvement of PLEKHM1 in osteoclastic vesicular transport and osteopetrosis in incisors absent rats and humans.J Clin Invest. 2007 Apr;117(4):919-30. doi: 10.1172/JCI30328. J Clin Invest. 2007. PMID: 17404618 Free PMC article.
References
-
- Blair HC, Teitelbaum SL, Ghiselli R, Gluck S: Osteoclastic bone resorption by a polarized vacuolar proton pump. Science 1989, 245:855-857 - PubMed
-
- Vaananen KH, Zhao H, Mulari M, Hallen JM: The cell biology of osteoclast function. J Cell Sci 2000, 113:377-381 - PubMed
-
- Blair HC, Schlesinger PH: The mechanism of osteoclast acidification. Rifkin BR Gay CV eds. Biology and Physiology of the Osteoclast. 1992:pp 259-287 CRC Press, Boca Raton
-
- Forgac M: Structure and properties of the vacuolar (H+)-ATPases. J Biol Chem 1999, 274:12951-12954 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

