Experimental autoimmune encephalomyelitis (EAE) in CCR2(-/-) mice: susceptibility in multiple strains
- PMID: 12507897
- PMCID: PMC1851120
- DOI: 10.1016/S0002-9440(10)63805-9
Experimental autoimmune encephalomyelitis (EAE) in CCR2(-/-) mice: susceptibility in multiple strains
Abstract
Chemokines are low molecular weight cytokines which act as chemoattractants for infiltrating cells bearing appropriate receptors (CCR) to sites of inflammation. It has been proposed that CCR2 on monocytes is responsible for their recruitment into the central nervous system (CNS) in experimental autoimmune encephalomyelitis (EAE), a model for multiple sclerosis, and two previous reports have described resistance of CCR2(-/-) mice to EAE. The present study examined three different mouse strains with CCR2 deletions for susceptibility to EAE. Animals were studied up to 4 months post-sensitization and were examined by neuropathology, RNase protection assay, in situ hybridization, and in vitro assays. All three strains were found to be susceptible to EAE: C57BL/6 x J129 and Balb c strains, 100%; and C57BL/6, 67%. Unusual in CNS lesions of CCR2(-/-) mice was an overabundance of neutrophils versus monocytes in wild-type animals. An attempt of the immune system to develop compensatory mechanisms for the lack of CCR2 was evidenced by a corresponding increase in mRNA for other chemokines and CCR. Inasmuch as neutrophils replaced monocytes and led to demyelination, our findings support the concept that promiscuity of chemokines and CCR was able to surmount the deletion of CCR2, still resulting in full expression of this autoimmune disease.
Figures




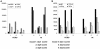

References
-
- Raine CS: Biology of disease. The analysis of autoimmune demyelination: its impact upon multiple sclerosis. Lab Invest 1984, 50:608-635 - PubMed
-
- Steinman L: Assessment to the utility of animal models for MS and demyelinating disease in the design of rational therapy. Neuron 1999, 24:511-514 - PubMed
-
- Raine CS: The lesion in multiple sclerosis and chronic relapsing experimental allergic encephalomyelitis: a structural comparison. Raine CS McFarland HF Tourtellotte WW eds. Multiple Sclerosis: Clinical and Pathogenetic Basis. 1997:pp 243-286 Chapman and Hall, London
-
- Cross AH, Cannella B, Brosnan CF, Raine CS: Homing to central nervous system vasculature by antigen-specific lymphocytes: I. localization of 14C-labeled cells during acute, chronic, and relapsing experimental allergic encephalomyelitis. Lab Invest 1990, 63:162-170 - PubMed
-
- Baggiolini M, Dahinden CA: CC chemokines in allergic inflammation. Immunol Today 1994, 15:127-133 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

