Two ZBP1 KH domains facilitate beta-actin mRNA localization, granule formation, and cytoskeletal attachment
- PMID: 12507992
- PMCID: PMC2172732
- DOI: 10.1083/jcb.200206003
Two ZBP1 KH domains facilitate beta-actin mRNA localization, granule formation, and cytoskeletal attachment
Abstract
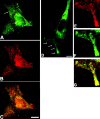
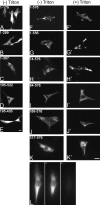
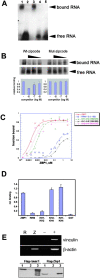
Chicken embryo fibroblasts (CEFs) localize beta-actin mRNA to their lamellae, a process important for the maintenance of cell polarity and motility. The localization of beta-actin mRNA requires a cis localization element (zipcode) and involves zipcode binding protein 1 (ZBP1), a protein that specifically binds to the zipcode. Both localize to the lamellipodia of polarized CEFs. ZBP1 and its homologues contain two NH2-terminal RNA recognition motifs (RRMs) and four COOH-terminal hnRNP K homology (KH) domains. By using ZBP1 truncations fused to GFP in conjunction with in situ hybridization analysis, we have determined that KH domains three and four were responsible for granule formation and cytoskeletal association. When the NH2 terminus was deleted, granules formed by the KH domains alone did not accumulate at the leading edge, suggesting a role for the NH2 terminus in targeting transport granules to their destination. RNA binding studies were used to show that the third and fourth KH domains, not the RRM domains, bind the zipcode of beta-actin mRNA. Overexpression of the four KH domains or certain subsets of these domains delocalized beta-actin mRNA in CEFs and inhibited fibroblast motility, demonstrating the importance of ZBP1 function in both beta-actin mRNA localization and cell motility.
Figures




References
-
- Barbarese, E., D.E. Koppel, M.P. Deutscher, C.L. Smith, K. Ainger, F. Morgan, and J.H. Carson. 1995. Protein translation components are colocalized in granules in oligodendrocytes. J. Cell Sci. 108:2781–2790. - PubMed
-
- Bashirullah, A., R.L. Cooperstock, and H.D. Lipshitz. 1998. RNA localization in development. Annu. Rev. Biochem. 67:335–394. - PubMed
-
- Broadus, J., and C.Q. Doe. 1997. Extrinsic cues, intrinsic cues and microfilaments regulate asymmetric protein localization in Drosophila neuroblasts. Curr. Biol. 7:827–835. - PubMed
-
- Carey, J., V. Cameron, P.L. de Haseth, and O.C. Uhlenbeck. 1983. Sequence-specific interaction of R17 coat protein with its ribonucleic acid binding site. Biochemistry. 22:2601–2610. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

