Roles of G beta gamma in membrane recruitment and activation of p110 gamma/p101 phosphoinositide 3-kinase gamma
- PMID: 12507995
- PMCID: PMC2172741
- DOI: 10.1083/jcb.200210115
Roles of G beta gamma in membrane recruitment and activation of p110 gamma/p101 phosphoinositide 3-kinase gamma
Abstract
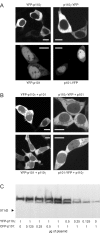
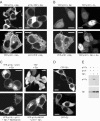
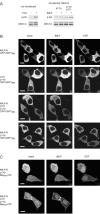
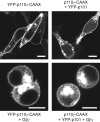
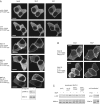
Receptor-regulated class I phosphoinositide 3-kinases (PI3K) phosphorylate the membrane lipid phosphatidylinositol (PtdIns)-4,5-P2 to PtdIns-3,4,5-P3. This, in turn, recruits and activates cytosolic effectors with PtdIns-3,4,5-P3-binding pleckstrin homology (PH) domains, thereby controlling important cellular functions such as proliferation, survival, or chemotaxis. The class IB p110 gamma/p101 PI3K gamma is activated by G beta gamma on stimulation of G protein-coupled receptors. It is currently unknown whether in living cells G beta gamma acts as a membrane anchor or an allosteric activator of PI3K gamma, and which role its noncatalytic p101 subunit plays in its activation by G beta gamma. Using GFP-tagged PI3K gamma subunits expressed in HEK cells, we show that G beta gamma recruits the enzyme from the cytosol to the membrane by interaction with its p101 subunit. Accordingly, p101 was found to be required for G protein-mediated activation of PI3K gamma in living cells, as assessed by use of GFP-tagged PtdIns-3,4,5-P3-binding PH domains. Furthermore, membrane-targeted p110 gamma displayed basal enzymatic activity, but was further stimulated by G beta gamma, even in the absence of p101. Therefore, we conclude that in vivo, G beta gamma activates PI3K gamma by a mechanism assigning specific roles for both PI3K gamma subunits, i.e., membrane recruitment is mediated via the noncatalytic p101 subunit, and direct stimulation of G beta gamma with p110 gamma contributes to activation of PI3K gamma.
Figures



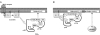
References
-
- Al-Aoukaty, A., B. Rolstad, and A.A. Maghazachi. 1999. Recruitment of pleckstrin and phosphoinositide 3-kinase γ into the cell membranes, and their association with Gβγ after activation of NK cells with chemokines. J. Immunol. 162:3249–3255. - PubMed
-
- Balla, T., T. Bondeva, and P. Várnai. 2000. How accurately can we image inositol lipids in living cells? Trends Pharmacol. Sci. 21:238–241. - PubMed
-
- Baier, R., T. Bondeva, R. Klinger, A. Bondev, and R. Wetzker. 1999. Retinoic acid induces selective expression of phosphoinositide 3-kinase γ in myelomonocytic U937 cells. Cell Growth Differ. 10:447–456. - PubMed
-
- Bondev, A., T. Bondeva, and R. Wetzker. 2001. Regulation of phosphoinositide 3-kinase γ protein kinase activity in vitro and in COS-7 cells. Signal Transduction. 1:79–85.
-
- Bondeva, T., L. Pirola, G. Bulgarelli-Leva, I. Rubio, R. Wetzker, and M.P. Wymann. 1998. Bifurcation of lipid and protein kinase signals of PI3Kγ to the protein kinases PKB and MAPK. Science. 282:293–296. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

