The 32-kilodalton subunit of replication protein A interacts with menin, the product of the MEN1 tumor suppressor gene
- PMID: 12509449
- PMCID: PMC151531
- DOI: 10.1128/MCB.23.2.493-509.2003
The 32-kilodalton subunit of replication protein A interacts with menin, the product of the MEN1 tumor suppressor gene
Abstract
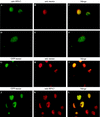
Menin is a 70-kDa protein encoded by MEN1, the tumor suppressor gene disrupted in multiple endocrine neoplasia type 1. In a yeast two-hybrid system based on reconstitution of Ras signaling, menin was found to interact with the 32-kDa subunit (RPA2) of replication protein A (RPA), a heterotrimeric protein required for DNA replication, recombination, and repair. The menin-RPA2 interaction was confirmed in a conventional yeast two-hybrid system and by direct interaction between purified proteins. Menin-RPA2 binding was inhibited by a number of menin missense mutations found in individuals with multiple endocrine neoplasia type 1, and the interacting regions were mapped to the N-terminal portion of menin and amino acids 43 to 171 of RPA2. This region of RPA2 contains a weak single-stranded DNA-binding domain, but menin had no detectable effect on RPA-DNA binding in vitro. Menin bound preferentially in vitro to free RPA2 rather than the RPA heterotrimer or a subcomplex consisting of RPA2 bound to the 14-kDa subunit (RPA3). However, the 70-kDa subunit (RPA1) was coprecipitated from HeLa cell extracts along with RPA2 by menin-specific antibodies, suggesting that menin binds to the RPA heterotrimer or a novel RPA1-RPA2-containing complex in vivo. This finding was consistent with the extensive overlap in the nuclear localization patterns of endogenous menin, RPA2, and RPA1 observed by immunofluorescence.
Figures








References
-
- Agarwal, S. K., S. C. Guru, C. Heppner, M. R. Erdos, R. M. Collins, S. Y. Park, S. Saggar, S. C. Chandrasekharappa, F. S. Collins, A. M. Spiegel, S. J. Marx, and A. L. Burns. 1999. Menin interacts with the AP1 transcription factor JunD and represses JunD-activated transcription. Cell 96:143-152. - PubMed
-
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
-
- Arnaudeau, C., C. Lundin, and T. Helleday. 2001. DNA double-strand breaks associated with replication forks are predominantly repaired by homologous recombination involving an exchange mechanism in mammalian cells. J. Mol. Biol. 307:1235-1245. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
