Regulation of RelA (p65) function by the large subunit of replication factor C
- PMID: 12509469
- PMCID: PMC151544
- DOI: 10.1128/MCB.23.2.721-732.2003
Regulation of RelA (p65) function by the large subunit of replication factor C
Abstract
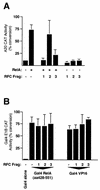
The RelA (p65) subunit of NF-kappaB is an important regulator of inflammation, proliferation, and apoptosis. We have discovered that the large subunit, p140, of replication factor C (RFC) can function as a regulator of RelA. RFC is a clamp loader, facilitating the addition and removal of proliferating-cell nuclear antigen from DNA during replication and repair but can also interact directly with the retinoblastoma tumor suppressor protein and the transcription factor C/EBPalpha. We find that RFC (p140) interacts with RelA both in vitro and in vivo and stimulates RelA transactivation. In contrast, coexpression of fragments of RFC (p140) that mediate the interaction with RelA results in transcriptional inhibition. The significance of this regulation was confirmed by using short interfering RNA oligonucleotides targeted to RFC (p140). Down regulation of endogenous RFC (p140) inhibits expression from a chromosomally integrated reporter plasmid induced by endogenous, TNF-alpha-activated NF-kappaB. Dominant negative fragments of RFC (p140) also cooperate with overexpressed RelA to induce cell death. Interestingly, RFC (p140) also interacts with the tumor suppressor p53. Taken together, these observations suggest that, in addition to its previously described function in DNA replication and repair, RFC (p140) has an important role as a regulator of transcription and NF-kappaB activity.
Figures




Similar articles
-
The large subunit of replication factor C interacts with the histone deacetylase, HDAC1.J Biol Chem. 2002 Aug 16;277(33):29550-4. doi: 10.1074/jbc.M200513200. Epub 2002 Jun 3. J Biol Chem. 2002. PMID: 12045192
-
PIAS3 suppresses NF-kappaB-mediated transcription by interacting with the p65/RelA subunit.J Biol Chem. 2004 Jun 4;279(23):24873-80. doi: 10.1074/jbc.M313018200. Epub 2004 Mar 26. J Biol Chem. 2004. PMID: 15140884
-
Centrosomal P4.1-associated protein is a new member of transcriptional coactivators for nuclear factor-kappaB.J Biol Chem. 2005 Apr 1;280(13):12430-7. doi: 10.1074/jbc.M410420200. Epub 2005 Jan 31. J Biol Chem. 2005. PMID: 15687488
-
A conserved interaction between the replicative clamp loader and DNA ligase in eukaryotes: implications for Okazaki fragment joining.J Biol Chem. 2004 Dec 31;279(53):55196-201. doi: 10.1074/jbc.M409250200. Epub 2004 Oct 23. J Biol Chem. 2004. PMID: 15502161
-
Overexpression of mouse p140 subunit of replication factor C accelerates cellular proliferation.Cell Growth Differ. 1996 Mar;7(3):319-26. Cell Growth Differ. 1996. PMID: 8838862
Cited by
-
Overexpression of RFC3 is correlated with ovarian tumor development and poor prognosis.Tumour Biol. 2014 Oct;35(10):10259-66. doi: 10.1007/s13277-014-2216-2. Epub 2014 Jul 17. Tumour Biol. 2014. PMID: 25030735
-
Recruitment of DNA repair synthesis machinery to sites of DNA damage/repair in living human cells.Nucleic Acids Res. 2007;35(9):2913-23. doi: 10.1093/nar/gkm115. Epub 2007 Apr 16. Nucleic Acids Res. 2007. PMID: 17439963 Free PMC article.
-
Evolutionary conserved regulation of HIF-1β by NF-κB.PLoS Genet. 2011 Jan 27;7(1):e1001285. doi: 10.1371/journal.pgen.1001285. PLoS Genet. 2011. PMID: 21298084 Free PMC article.
-
Control of Genome Integrity by RFC Complexes; Conductors of PCNA Loading onto and Unloading from Chromatin during DNA Replication.Genes (Basel). 2017 Jan 26;8(2):52. doi: 10.3390/genes8020052. Genes (Basel). 2017. PMID: 28134787 Free PMC article. Review.
-
Disruption of the moonlighting function of CTF18 in a patient with T-lymphopenia.Front Immunol. 2025 Feb 14;16:1539848. doi: 10.3389/fimmu.2025.1539848. eCollection 2025. Front Immunol. 2025. PMID: 40028343 Free PMC article.
References
-
- Agalioti, T., S. Lomvardas, B. Parekh, J. M. Yie, T. Maniatis, and D. Thanos. 2000. Ordered recruitment of chromatin modifying and general transcription factors to the IFN-β promoter. Cell 103:667-678. - PubMed
-
- Anderson, L. A., and N. D. Perkins. 2002. The large subunit of replication factor C interacts with the histone deacetylase, HDAC1. J. Biol. Chem. 277:29550-29554. - PubMed
-
- Barkett, M., and T. D. Gilmore. 1999. Control of apoptosis by Rel/NF-κB transcription factors. Oncogene 18:6910-6924. - PubMed
-
- Barnes, P. J., and M. Karin. 1997. Mechanisms of disease: nuclear factor κB, a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 336:1066-1071. - PubMed
-
- Beg, A. A., W. C. Sha, R. T. Bronson, S. Ghosh, and D. Baltimore. 1995. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature 376:167-170. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
