Regulation of cardiac excitation-contraction coupling by action potential repolarization: role of the transient outward potassium current (I(to))
- PMID: 12509475
- PMCID: PMC2342473
- DOI: 10.1113/jphysiol.2002.026468
Regulation of cardiac excitation-contraction coupling by action potential repolarization: role of the transient outward potassium current (I(to))
Abstract
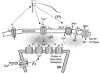
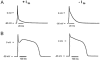
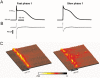
The cardiac action potential (AP) is critical for initiating and coordinating myocyte contraction. In particular, the early repolarization period of the AP (phase 1) strongly influences the time course and magnitude of the whole-cell intracellular Ca(2+) transient by modulating trans-sarcolemmal Ca(2+) influx through L-type Ca(2+) channels (I(Ca,L)) and Na-Ca exchangers (I(Ca,NCX)). The transient outward potassium current (I(to)) has kinetic properties that make it especially effective in modulating the trajectory of phase 1 repolarization and thereby cardiac excitation-contraction coupling (ECC). The magnitude of I(to) varies greatly during cardiac development, between different regions of the heart, and is invariably reduced as a result of heart disease, leading to corresponding variations in ECC. In this article, we review evidence supporting a modulatory role of I(to) in ECC through its influence on I(Ca,L), and possibly I(Ca,NCX). We also discuss differential effects of I(to) on ECC between different species, between different regions of the heart and in heart disease.
Figures
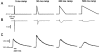
Similar articles
-
Phase 1 repolarization rate defines Ca2+ dynamics and contractility on intact mouse hearts.J Gen Physiol. 2019 Jun 3;151(6):771-785. doi: 10.1085/jgp.201812269. Epub 2019 Apr 18. J Gen Physiol. 2019. PMID: 31000581 Free PMC article.
-
Modulation of Ca(2+) release in cardiac myocytes by changes in repolarization rate: role of phase-1 action potential repolarization in excitation-contraction coupling.Circ Res. 2002 Feb 8;90(2):165-73. doi: 10.1161/hh0202.103315. Circ Res. 2002. PMID: 11834709
-
Transient outward potassium current and Ca2+ homeostasis in the heart: beyond the action potential.Braz J Med Biol Res. 2006 Mar;39(3):393-403. doi: 10.1590/s0100-879x2006000300010. Epub 2006 Feb 22. Braz J Med Biol Res. 2006. PMID: 16501819 Review.
-
Intracellular [Na(+)] modulates synergy between Na(+)/Ca (2+) exchanger and L-type Ca (2+) current in cardiac excitation-contraction coupling during action potentials.Basic Res Cardiol. 2011 Nov;106(6):967-77. doi: 10.1007/s00395-011-0202-z. Epub 2011 Jul 21. Basic Res Cardiol. 2011. PMID: 21779914
-
Na(+)--Ca2+ exchange in the regulation of cardiac excitation-contraction coupling.Cardiovasc Res. 2005 Aug 1;67(2):198-207. doi: 10.1016/j.cardiores.2005.04.031. Cardiovasc Res. 2005. PMID: 15935336 Review.
Cited by
-
Inhibition of Kv4.3 potassium channels by trazodone.Naunyn Schmiedebergs Arch Pharmacol. 2013 Aug;386(8):711-9. doi: 10.1007/s00210-013-0870-3. Epub 2013 Apr 25. Naunyn Schmiedebergs Arch Pharmacol. 2013. PMID: 23615873
-
Simvastatin modulates remodeling of Kv4.3 expression in rat hypertrophied cardiomyocytes.Int J Biol Sci. 2012;8(2):236-48. doi: 10.7150/ijbs.8.236. Epub 2012 Jan 6. Int J Biol Sci. 2012. PMID: 22253567 Free PMC article.
-
K+ currents activated by depolarization in cardiac fibroblasts.Biophys J. 2005 Jun;88(6):3924-35. doi: 10.1529/biophysj.104.054429. Epub 2005 Mar 11. Biophys J. 2005. PMID: 15764658 Free PMC article.
-
Analysis of the contribution of I(to) to repolarization in canine ventricular myocardium.Br J Pharmacol. 2011 Sep;164(1):93-105. doi: 10.1111/j.1476-5381.2011.01331.x. Br J Pharmacol. 2011. PMID: 21410683 Free PMC article.
-
Phase 1 repolarization rate defines Ca2+ dynamics and contractility on intact mouse hearts.J Gen Physiol. 2019 Jun 3;151(6):771-785. doi: 10.1085/jgp.201812269. Epub 2019 Apr 18. J Gen Physiol. 2019. PMID: 31000581 Free PMC article.
References
-
- Ahmmed GU, Dong PH, Song G, Ball NA, Xu Y, Walsh RA, Chiamvimonvat N. Changes in Ca(2+) cycling proteins underlie cardiac action potential prolongation in a pressure-overloaded guinea pig model with cardiac hypertrophy and failure. Circ Res. 2000;86:558–570. - PubMed
-
- Arai M, Alpert NR, MacLennan DH, Barton P, Periasamy M. Alterations in sarcoplasmic reticulum gene expression in human heart failure. A possible mechanism for alterations in systolic and diastolic properties of the failing myocardium. Circ Res. 1993;72:463–469. - PubMed
-
- Bassani JW, Yuan W, Bers DM. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol. 1995;268:C1313–1319. - PubMed
-
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002a;415:198–205. - PubMed
-
- Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht, Netherlands: Kluwer Academic Press; 2002b.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

