Local calcium gradients during excitation-contraction coupling and alternans in atrial myocytes
- PMID: 12509476
- PMCID: PMC2342467
- DOI: 10.1113/jphysiol.2002.025239
Local calcium gradients during excitation-contraction coupling and alternans in atrial myocytes
Abstract
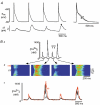
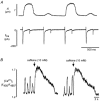
Subcellular Ca(2+) signalling during normal excitation-contraction (E-C) coupling and during Ca(2+) alternans was studied in atrial myocytes using fast confocal microscopy and measurement of Ca(2+) currents (I(Ca)). Ca(2+) alternans, a beat-to-beat alternation in the amplitude of the [Ca(2+)](i) transient, causes electromechanical alternans, which has been implicated in the generation of cardiac fibrillation and sudden cardiac death. Cat atrial myocytes lack transverse tubules and contain sarcoplasmic reticulum (SR) of the junctional (j-SR) and non-junctional (nj-SR) types, both of which have ryanodine-receptor calcium release channels. During E-C coupling, Ca(2+) entering through voltage-gated membrane Ca(2+) channels (I(Ca)) triggers Ca(2+) release at discrete peripheral j-SR release sites. The discrete Ca(2+) spark-like increases of [Ca(2+)](i) then fuse into a peripheral 'ring' of elevated [Ca(2+)](i), followed by propagation (via calcium-induced Ca(2+) release, CICR) to the cell centre, resulting in contraction. Interrupting I(Ca) instantaneously terminates j-SR Ca(2+) release, whereas nj-SR Ca(2+) release continues. Increasing the stimulation frequency or inhibition of glycolysis elicits Ca(2+) alternans. The spatiotemporal [Ca(2+)](i) pattern during alternans shows marked subcellular heterogeneities including longitudinal and transverse gradients of [Ca(2+)](i) and neighbouring subcellular regions alternating out of phase. Moreover, focal inhibition of glycolysis causes spatially restricted Ca(2+) alternans, further emphasising the local character of this phenomenon. When two adjacent regions within a myocyte alternate out of phase, delayed propagating Ca(2+) waves develop at their border. In conclusion, the results demonstrate that (1) during normal E-C coupling the atrial [Ca(2+)](i) transient is the result of the spatiotemporal summation of Ca(2+) release from individual release sites of the peripheral j-SR and the central nj-SR, activated in a centripetal fashion by CICR via I(Ca) and Ca(2+) release from j-SR, respectively, (2) Ca(2+) alternans is caused by subcellular alterations of SR Ca(2+) release mediated, at least in part, by local inhibition of energy metabolism, and (3) the generation of arrhythmogenic Ca(2+) waves resulting from heterogeneities in subcellular Ca(2+) alternans may constitute a novel mechanism for the development of cardiac dysrhythmias.
Figures

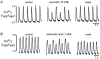

References
-
- Badeer HS, Ryo UY, Gassner WF, Kass EJ, Cavaluzzi J, Gilbert JL, Brooks CM. Factors affecting pulsus alternans in the rapidly driven heart and papillary muscle. Am J Physiol. 1967;213:1095–1101. - PubMed
-
- Berger RD. Repolarization alternans. Toward a unifying theory of reentrant arrhythmia induction. Circ Res. 2000;87:1083–1084. - PubMed
-
- Berlin JR. Spatiotemporal changes of Ca2+ during electrically evoked contractions in atrial and ventricular cells. Am J Physiol. 1995;269:H1165–1170. - PubMed
-
- Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht, The Netherlands: Kluwer Academic Publishers Group; 2001.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

