Distribution, subcellular localization and ontogeny of ASIC1 in the mammalian central nervous system
- PMID: 12509480
- PMCID: PMC2342460
- DOI: 10.1113/jphysiol.2002.030692
Distribution, subcellular localization and ontogeny of ASIC1 in the mammalian central nervous system
Abstract
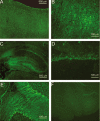
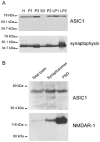
The acid-sensitive ion channel ASIC1 is a proton-gated ion channel from the mammalian nervous system. Its expression in sensory neurons and activation by low extracellular pH suggest that ASIC is involved in transmitting nociceptive impulses produced by the acidification caused by injury or inflammation. However, ASIC1 expression is not restricted to sensory neurons. To understand the functional role of ASIC1 in the CNS we investigated its expression and subcellular distribution therein. In particular, we examined the presence of ASIC1 in domains where the local pH may drop sufficiently to activate ASIC1 under physiological conditions. Immunostaining with specific antibodies revealed broad expression of ASIC1 in many areas of the adult rat brain including the cerebral cortex, hippocampus and cerebellum. Within cells, ASIC1 was found predominantly throughout the soma and along the branches of axons and dendrites. ASIC1 was not enriched in the microdomains where pH may reach low values, such as in synaptic vesicles or synaptic membranes. Pre- or postsynaptic ASIC1 was not gated by synaptic activity in cultured hippocampal neurons. Blockage or desensitization of ASIC1 with amiloride or pH 6.7, respectively, did not modify postsynaptic currents. Finally, the ontogeny of ASIC1 in mouse brain revealed constant levels of expression of ASIC1 protein from embryonic day 12 to the postnatal period, indicating an early and almost constant level of expression of ASIC1 during brain development.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

