Pacemaker frequency is increased by sodium nitroprusside in the guinea pig gastric antrum
- PMID: 12509488
- PMCID: PMC2342478
- DOI: 10.1113/jphysiol.2002.027607
Pacemaker frequency is increased by sodium nitroprusside in the guinea pig gastric antrum
Abstract
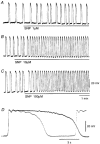
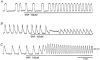
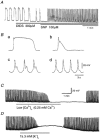
In the guinea pig gastric antrum, the effects of sodium nitroprusside (SNP), an NO donor, on pacemaker potentials were investigated in the presence of nifedipine. The pacemaker potentials consisted of primary and plateau components; SNP (> 1 microM) increased the frequency of occurrence of these pacemaker potentials, while inhibiting the plateau component. 1H-[1,2,4]-Oxadiazole [4,3-a] quinoxalin-1-one, an inhibitor of guanylate cyclase, had no effect on the excitatory actions of SNP on the frequency of pacemaker potentials. Other types of NO donor, (+/-)-S-nitroso-N-acetylpenicillamine, 3-morpholino-sydnonimine and 8-bromoguanosine 3'5'-cyclic monophosphate had no excitatory effect on pacemaker activity. Forskolin, an activator of adenylate cyclase, or 4,4'-diisothiocyano-stilbene-2,2'-disulphonic acid, an inhibitor of the Ca(2+)-activated Cl(-) channel, strongly attenuated the generation of pacemaker potentials, and SNP added in the presence of these chemicals restored the generation of pacemaker potentials. The pacemaker potentials evoked by SNP were abolished in low-Ca(2+) solution or by membrane depolarization with high-K(+) solution. The SNP-induced generation of pacemaker potentials was not prevented by cyclopiazonic acid, an inhibitor of internal Ca(2+)-ATPase, but was limited to a transient burst by iodoacetic acid, an inhibitor of glycolysis, carbonyl cyanide m-chlorophenyl-hydrazone, a mitochondrial protonophore, or 1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid acetoxymethyl ester, an intracellular Ca(2+) chelator. These results suggest that the SNP-induced increase in the frequency of pacemaker potentials is related to the elevated intracellular Ca(2+) concentrations due to release from mitochondria, and these actions may be independent of the activation of guanylate cyclase.
Figures

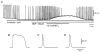




Similar articles
-
Components of pacemaker potentials recorded from the guinea pig stomach antrum.Pflugers Arch. 2002 Nov;445(2):202-17. doi: 10.1007/s00424-002-0884-z. Epub 2002 Oct 25. Pflugers Arch. 2002. PMID: 12457241
-
Properties of unitary potentials recorded from myenteric interstitial cells of Cajal distributed in the guinea-pig gastric antrum.J Smooth Muscle Res. 2002 Dec;38(6):165-79. doi: 10.1540/jsmr.38.165. J Smooth Muscle Res. 2002. PMID: 12713023
-
Effects of nitric oxide (NO) and NO donors on the membrane conductance of circular smooth muscle cells of the guinea-pig proximal colon.Br J Pharmacol. 1996 Aug;118(7):1605-14. doi: 10.1111/j.1476-5381.1996.tb15581.x. Br J Pharmacol. 1996. PMID: 8842421 Free PMC article.
-
Effect of nitric oxide donors and noradrenaline on Ca2+ release sites and global intracellular Ca2+ in myocytes from guinea-pig small mesenteric arteries.J Physiol. 2002 Feb 15;539(Pt 1):25-39. doi: 10.1113/jphysiol.2001.012978. J Physiol. 2002. PMID: 11850499 Free PMC article.
-
Electrophysiological properties of gastric pacemaker potentials.J Smooth Muscle Res. 2003 Oct;39(5):163-73. doi: 10.1540/jsmr.39.163. J Smooth Muscle Res. 2003. PMID: 14695027 Review.
Cited by
-
Modulation of slow waves by hyperpolarization with potassium channel openers in antral smooth muscle of the guinea-pig stomach.J Physiol. 2003 Apr 1;548(Pt 1):175-89. doi: 10.1113/jphysiol.2002.035550. Epub 2003 Feb 21. J Physiol. 2003. PMID: 12598588 Free PMC article.
-
Cellular mechanism of the voltage-dependent change in slow potentials generated in circular smooth muscle of the guinea-pig gastric corpus.J Physiol. 2008 Nov 15;586(22):5521-36. doi: 10.1113/jphysiol.2008.160531. Epub 2008 Sep 25. J Physiol. 2008. PMID: 18818248 Free PMC article.
-
Properties of pacemaker potentials recorded from myenteric interstitial cells of Cajal distributed in the mouse small intestine.J Physiol. 2003 Dec 15;553(Pt 3):803-18. doi: 10.1113/jphysiol.2003.051334. Epub 2003 Oct 17. J Physiol. 2003. PMID: 14565995 Free PMC article.
-
Effects of temperature on pacemaker potentials in the mouse small intestine.Pflugers Arch. 2007 May;454(2):263-75. doi: 10.1007/s00424-006-0201-3. Epub 2007 Jan 18. Pflugers Arch. 2007. PMID: 17235578
-
Voltage-dependent Ca Current Identified in Freshly Isolated Interstitial Cells of Cajal (ICC) of Guinea-pig Stomach.Korean J Physiol Pharmacol. 2008 Dec;12(6):323-30. doi: 10.4196/kjpp.2008.12.6.323. Epub 2008 Dec 31. Korean J Physiol Pharmacol. 2008. PMID: 19967074 Free PMC article.
References
-
- Arnaudeau S, Kelley WL, Walsh JV, Jr, Demaurex N. Mitochondria recycle Ca2+ to the endoplasmic reticulum and prevent the depletion of neighboring endoplasmic reticulum regions. J Biol Chem. 2001;276:29430–29439. - PubMed
-
- Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. - PubMed
-
- Bragadin M, Viola ER. Ni2+ as a competitive inhibitor of calcium transport in mitochondria. J Inorg Biochem. 1997;66:227–229. - PubMed
-
- Carvajal JA, Germain AM, Huidobro-Toro JP, Weiner CP. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J Cell Physiol. 2000;184:409–420. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

